Pet-1 is required across different stages of life to regulate serotonergic function
- PMID: 20818386
- PMCID: PMC2947586
- DOI: 10.1038/nn.2623
Pet-1 is required across different stages of life to regulate serotonergic function
Abstract
Transcriptional cascades are required for the specification of serotonin (5-HT) neurons and behaviors modulated by 5-HT. Several cascade factors are expressed throughout the lifespan, which suggests that their control of behavior might not be temporally restricted to programming normal numbers of 5-HT neurons. We used new mouse conditional targeting approaches to investigate the ongoing requirements for Pet-1 (also called Fev), a cascade factor that is required for the initiation of 5-HT synthesis, but whose expression persists into adulthood. We found that Pet-1 was required after the generation of 5-HT neurons for multiple steps in 5-HT neuron maturation, including axonal innervation of the somatosensory cortex, expression of appropriate firing properties, and the expression of the Htr1a and Htr1b autoreceptors. Pet-1 was still required in adult 5-HT neurons to preserve normal anxiety-related behaviors through direct autoregulated control of serotonergic gene expression. These findings indicate that Pet-1 is required across the lifespan of the mouse and that behavioral pathogenesis can result from both developmental and adult-onset alterations in serotonergic transcription.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

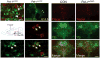
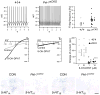
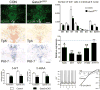



References
-
- Ansorge MS, Hen R, Gingrich JA. Neurodevelopmental origins of depressive disorders. Current opinion in pharmacology. 2007;7:8–17. - PubMed
-
- Vitalis T, Cases O, Passemard S, Callebert J, Parnavelas JG. Embryonic depletion of serotonin affects cortical development. Eur J Neurosci. 2007;26 :331–344. - PubMed
-
- Bonnin A, Torii M, Wang L, Rakic P, Levitt P. Serotonin modulates the response of embryonic thalamocortical axons to netrin-1. Nat Neurosci. 2007;10:588–597. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

