The RNA helicase p68 modulates expression and function of the Δ133 isoform(s) of p53, and is inversely associated with Δ133p53 expression in breast cancer
- PMID: 20818423
- PMCID: PMC3016604
- DOI: 10.1038/onc.2010.381
The RNA helicase p68 modulates expression and function of the Δ133 isoform(s) of p53, and is inversely associated with Δ133p53 expression in breast cancer
Abstract
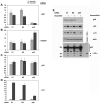
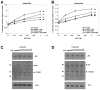
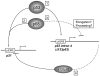
The RNA helicase p68 is a potent co-activator of p53-dependent transcription in response to DNA damage. Previous independent studies have indicated that p68 and the Δ133p53 isoforms, which modulate the function of full-length p53, are aberrantly expressed in breast cancers. Here we identify a striking inverse association of p68 and Δ133p53 expression in primary breast cancers. Consistent with these findings, small interfering RNA depletion of p68 in cell lines results in a p53-dependant increase of Δ133p53 in response to DNA damage, suggesting that increased Δ133p53 expression could result from downregulation of p68 and provide a potential mechanistic explanation for our observations in breast cancer. Δ133p53α, which has been shown to negatively regulate the function of full-length p53, reciprocally inhibits the ability of p68 to stimulate p53-dependent transcription from the p21 promoter, suggesting that Δ133p53α may be competing with p68 to regulate p53 function. This hypothesis is underscored by our observations that p68 interacts with the C-terminal domain of p53, co-immunoprecipitates 133p53α from cell extracts and interacts only with p53 molecules that are able to form tetramers. These data suggest that p68, p53 and 133p53α may form part of a complex feedback mechanism to regulate the expression of Δ133p53, with consequent modification of p53-mediated transcription, and may modulate the function of p53 in breast and other cancers that harbour wild-type p53.
Figures



Similar articles
-
P68 RNA helicase as a molecular target for cancer therapy.J Exp Clin Cancer Res. 2014 Aug 24;33(1):64. doi: 10.1186/s13046-014-0064-y. J Exp Clin Cancer Res. 2014. PMID: 25150365 Free PMC article. Review.
-
The RNA helicase/transcriptional co-regulator, p68 (DDX5), stimulates expression of oncogenic protein kinase, Polo-like kinase-1 (PLK1), and is associated with elevated PLK1 levels in human breast cancers.Cell Cycle. 2014;13(9):1413-23. doi: 10.4161/cc.28415. Epub 2014 Mar 6. Cell Cycle. 2014. PMID: 24626184 Free PMC article.
-
The RNA helicase p68 (DDX5) is selectively required for the induction of p53-dependent p21 expression and cell-cycle arrest after DNA damage.Oncogene. 2013 Jul 18;32(29):3461-9. doi: 10.1038/onc.2012.426. Epub 2012 Sep 17. Oncogene. 2013. PMID: 22986526 Free PMC article.
-
Analysis of the RNA helicase p68 (Ddx5) as a transcriptional regulator.Methods Mol Biol. 2010;587:265-79. doi: 10.1007/978-1-60327-355-8_19. Methods Mol Biol. 2010. PMID: 20225156
-
The role of DEAD-box RNA helicase p68 (DDX5) in the development and treatment of breast cancer.J Cell Physiol. 2019 May;234(5):5478-5487. doi: 10.1002/jcp.26912. Epub 2018 Nov 11. J Cell Physiol. 2019. PMID: 30417346 Review.
Cited by
-
The Δ133p53 Isoforms, Tuners of the p53 Pathway.Cancers (Basel). 2020 Nov 18;12(11):3422. doi: 10.3390/cancers12113422. Cancers (Basel). 2020. PMID: 33218139 Free PMC article. Review.
-
p53 and its isoforms in DNA double-stranded break repair.J Zhejiang Univ Sci B. 2019 Jun;20(6):457-466. doi: 10.1631/jzus.B1900167. J Zhejiang Univ Sci B. 2019. PMID: 31090271 Free PMC article. Review.
-
P68 RNA helicase as a molecular target for cancer therapy.J Exp Clin Cancer Res. 2014 Aug 24;33(1):64. doi: 10.1186/s13046-014-0064-y. J Exp Clin Cancer Res. 2014. PMID: 25150365 Free PMC article. Review.
-
A functional interplay between Δ133p53 and ΔNp63 in promoting glycolytic metabolism to fuel cancer cell proliferation.Oncogene. 2018 Apr;37(16):2150-2164. doi: 10.1038/s41388-017-0117-8. Epub 2018 Jan 26. Oncogene. 2018. PMID: 29371679
-
DDX5/p68 associated lncRNA LOC284454 is differentially expressed in human cancers and modulates gene expression.RNA Biol. 2018 Feb 1;15(2):214-230. doi: 10.1080/15476286.2017.1397261. Epub 2017 Dec 11. RNA Biol. 2018. PMID: 29227193 Free PMC article.
References
-
- Avery-Kiejda KA, Zhang XD, Adams LJ, Scott RJ, Vojtesek B, Lane DP, et al. Small molecular weight variants of p53 are expressed in human melanoma cells and are induced by the DNA-damaging agent cisplatin. Clin Cancer Res. 2008;14:1659–68. - PubMed
-
- Balakrishnan SK, Gross DS. The tumor suppressor p53 associates with gene coding regions and co-traverses with elongating RNA polymerase II in an in vivo model. Oncogene. 2008;27:2661–72. - PubMed
-
- Bertheau P, Espie M, Turpin E, Lehmann J, Plassa LF, Varna M, et al. TP53 status and response to chemotherapy in breast cancer. Pathobiology. 2008;75:132–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

