Effects of titanium dioxide nanoparticle exposure on neuroimmune responses in rat airways
- PMID: 20818535
- PMCID: PMC3655524
- DOI: 10.1080/15287394.2010.497436
Effects of titanium dioxide nanoparticle exposure on neuroimmune responses in rat airways
Abstract
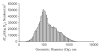
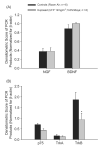
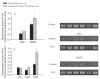
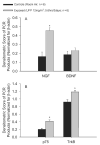
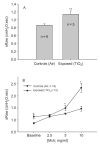
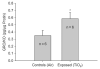
Exposure to ambient nanoparticles (defined as particulate matter [PM] having one dimension <100 nm) is associated with increased risk of childhood and adult asthma. Nanomaterials feature a smaller aerodynamic diameter and a higher surface area per unit mass ratio compared to fine or coarse-sized particles, resulting in greater lung deposition efficiency and an increased potential for biological interaction. The neurotrophins nerve growth factor and brain-derived neurotrophic factor are key regulatory elements of neuronal development and responsiveness of airway sensory neurons. Changes in their expression are associated with bronchoconstriction, airway hyperresponsiveness, and airway inflammation. The neurogenic-mediated control of airway responses is a key pathophysiological mechanism of childhood asthma. However, the effects of nanoparticle exposure on neurotrophin-driven airway responses and their potential role as a predisposing factor for developing asthma have not been clearly elucidated. In this study, in vivo inhalation exposure to titanium dioxide nanoparticles (12 mg/m(3); 5.6 h/d for 3 d) produced upregulation of lung neurotrophins in weanling (2-wk-old) and newborn (2-d-old) rats but not in adult (12-wk-old) animals compared to controls. This effect was associated with increased airway responsiveness and upregulation of growth-related oncogene/keratine-derived chemokine (GRO/KC; CXCL1, rat equivalent of human interleukin [IL]-8) in bronchoalveolar lavage fluid. These data show for the first time that exposure to nanoparticulate upregulates the expression of lung neurotrophins in an age-dependent fashion and that this effect is associated with airway hyperresponsiveness and inflammation. These results suggest the presence of a critical window of vulnerability in earlier stages of lung development, which may lead to a higher risk of developing asthma.
Figures
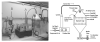

References
-
- Atkinson RW, Anderson HR, Sunyer J, Ayres J, Baccini M, Vonk JM, Boumghar A, Forastiere F, Forsberg B, Touloumi G, Schwartz J, Katsouyanni K. Acute effects of particulate air pollution on respiratory admissions: Results from APHEA 2 project. Air Pollution and Health: A European Approach. Am. J. Respir. Crit. Care Med. 2001;164:1860–1866. - PubMed
-
- Bedeschi E, Campari C, Candela S, Collini G, Caranci N, Frasca G, Galassi C, Francesca G, Vigotti MA. Urban air pollution and respiratory emergency visits at pediatric unit, Reggio Emilia, Italy. J. Toxicol. Environ. Health A. 2007;70:261–265. - PubMed
-
- BeruBe K, Balharry D, Sexton K, Koshy L, Jones T. Combustion-derived nanoparticles: Mechanisms of pulmonary toxicity. Clin. Exp. Pharmacol. Physiol. 2007;34:1044–1050. - PubMed
-
- Braun A, Appel E, Baruch R, Herz U, Botchkarev V, Paus R, Brodie C, Renz H. Role of nerve growth factor in a mouse model of allergic airway inflammation and asthma. Eur. J. Immunol. 1998;28:3240–3251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
