Ontogeny of the kidney and renal developmental markers in the rhesus monkey (Macaca mulatta)
- PMID: 20818613
- PMCID: PMC3163141
- DOI: 10.1002/ar.21242
Ontogeny of the kidney and renal developmental markers in the rhesus monkey (Macaca mulatta)
Abstract
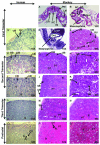
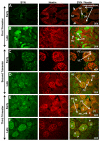
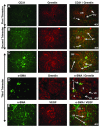
Nonhuman primates share many developmental similarities with humans, thus they provide an important preclinical model for understanding the ontogeny of biomarkers of kidney development and assessing new cell-based therapies to treat human disease. To identify morphological and developmental changes in protein and RNA expression patterns during nephrogenesis, immunohistochemistry and quantitative real-time PCR were used to assess temporal and spatial expression of WT1, Pax2, Nestin, Synaptopodin, alpha-smooth muscle actin (α-SMA), CD31, vascular endothelial growth factor (VEGF), and Gremlin. Pax2 was expressed in the condensed mesenchyme surrounding the ureteric bud and in the early renal vesicle. WT1 and Nestin were diffusely expressed in the metanephric mesenchyme, and expression increased as the Pax2-positive condensed mesenchyme differentiated. The inner cleft of the tail of the S-shaped body contained the podocyte progenitors (visceral epithelium) that were shown to express Pax2, Nestin, and WT1 in the early second trimester. With maturation of the kidney, Pax2 expression diminished in these structures, but was retained in cells of the parietal epithelium, and as WT1 expression was upregulated. Mature podocytes expressing WT1, Nestin, and Synaptopodin were observed from the mid-third trimester through adulthood. The developing glomerulus was positive for α-SMA (vascular smooth muscle) and Gremlin (mesangial cells), CD31 (glomerular endothelium), and VEGF (endothelium), and showed loss of expression of these markers as glomerular maturation was completed. These data form the basis for understanding nephrogenesis in the rhesus monkey and will be useful in translational studies that focus on embryonic stem and other progenitor cell populations for renal tissue engineering and repair.
Figures




References
-
- Agrawal R, Tran U, Wessely O. Expression and functional analysis of miRNAs in kidney development. Dev Biol. 2008;319:605. doi:10.1016/j.ydbio.2008.05.447.
-
- Armstrong JF, Pritchard-Jones K, Bickmore WA, Hastie ND, Bard JBL. The expression of the Wilms’ tumour gene, WT1, in the developing mammalian embryo. Mech Dev. 1992;40:85–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

