Inhibition of mutated, activated BRAF in metastatic melanoma
- PMID: 20818844
- PMCID: PMC3724529
- DOI: 10.1056/NEJMoa1002011
Inhibition of mutated, activated BRAF in metastatic melanoma
Abstract
Background: The identification of somatic mutations in the gene encoding the serine-threonine protein kinase B-RAF (BRAF) in the majority of melanomas offers an opportunity to test oncogene-targeted therapy for this disease.
Methods: We conducted a multicenter, phase 1, dose-escalation trial of PLX4032 (also known as RG7204), an orally available inhibitor of mutated BRAF, followed by an extension phase involving the maximum dose that could be administered without adverse effects (the recommended phase 2 dose). Patients received PLX4032 twice daily until they had disease progression. Pharmacokinetic analysis and tumor-response assessments were conducted in all patients. In selected patients, tumor biopsy was performed before and during treatment to validate BRAF inhibition.
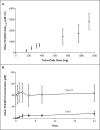
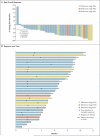
Results: A total of 55 patients (49 of whom had melanoma) were enrolled in the dose-escalation phase, and 32 additional patients with metastatic melanoma who had BRAF with the V600E mutation were enrolled in the extension phase. The recommended phase 2 dose was 960 mg twice daily, with increases in the dose limited by grade 2 or 3 rash, fatigue, and arthralgia. In the dose-escalation cohort, among the 16 patients with melanoma whose tumors carried the V600E BRAF mutation and who were receiving 240 mg or more of PLX4032 twice daily, 10 had a partial response and 1 had a complete response. Among the 32 patients in the extension cohort, 24 had a partial response and 2 had a complete response. The estimated median progression-free survival among all patients was more than 7 months.
Conclusions: Treatment of metastatic melanoma with PLX4032 in patients with tumors that carry the V600E BRAF mutation resulted in complete or partial tumor regression in the majority of patients. (Funded by Plexxikon and Roche Pharmaceuticals.)
Figures

Comment in
-
Melanoma--an unlikely poster child for personalized cancer therapy.N Engl J Med. 2010 Aug 26;363(9):876-8. doi: 10.1056/NEJMe1005370. N Engl J Med. 2010. PMID: 20818849 No abstract available.
-
Inhibition of mutated BRAF in melanoma.N Engl J Med. 2010 Dec 2;363(23):2261; author reply 2261-2. doi: 10.1056/NEJMc1010755. N Engl J Med. 2010. PMID: 21121840 No abstract available.
-
Melanoma-targeted therapy based on V600E BRAF mutation.Biomark Med. 2010 Dec;4(6):792. Biomark Med. 2010. PMID: 21174863 No abstract available.
-
When are signal transduction targeted therapies acting as immunotherapy?Cancer Biol Ther. 2015;16(5):645-7. doi: 10.1080/15384047.2015.1030553. Cancer Biol Ther. 2015. PMID: 25807058 Free PMC article.
References
-
- Comis RL. DTIC (NSC-45388) in malignant melanoma: a perspective. Cancer Treat Rep. 1976;60:165–76. - PubMed
-
- Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–16. - PubMed
-
-
Middleton MR, Grob JJ, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18:158–66. [Erratum, J Clin Oncol 2000;18:2351.]
-
-
- Bedikian AY, Millward M, Pehamberger H, et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. J Clin Oncol. 2006;24:4738–45. - PubMed
-
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
