Immediate versus delayed quitting and rates of relapse among smokers treated successfully with varenicline, bupropion SR or placebo
- PMID: 20819082
- PMCID: PMC2991770
- DOI: 10.1111/j.1360-0443.2010.03058.x
Immediate versus delayed quitting and rates of relapse among smokers treated successfully with varenicline, bupropion SR or placebo
Abstract
Aims: We assessed to what degree smokers who fail to quit on the target quit date (TQD) or lapse following TQD eventually achieve success with continued treatment.
Design: A secondary analysis of pooled data of successful quitters treated with varenicline (306 of 696), bupropion (199 of 671) and placebo (121 of 685) from two identically-designed clinical trials of varenicline versus bupropion sustained-release and placebo.
Setting: Multiple research centers in the US.
Participants: Adult smokers (n==2052) randomized to 12 weeks drug treatment plus 40 weeks follow-up.
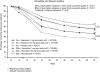
Measurement: The primary end-point for the trials was continuous abstinence for weeks 9-12. TQD was day 8. Two patterns of successful quitting were identified. Immediate quitters (IQs) were continuously abstinent for weeks 2-12. Delayed quitters (DQs) smoked during 1 or more weeks for weeks 2-8.
Findings: Cumulative continuous abstinence (IQs + DQs) increased for all treatments during weeks 3-8. Overall IQs and DQs for varenicline were (24%; 20%) versus bupropion (18.0%, P=0.007; 11.6%, P<0.001) or placebo (10.2%, P<0.001; 7.5%, P<0.001). However, DQs as a proportion of successful quitters was similar for all treatments (varenicline 45%; bupropion 39%; placebo 42%) and accounted for approximately one-third of those remaining continuously abstinent for weeks 9-52. No gender differences were observed by quit pattern. Post-treatment relapse was similar across groups.
Conclusions: Our data support continuing cessation treatments without interruption for smokers motivated to remain in the quitting process despite lack of success early in the treatment.
Trial registration: ClinicalTrials.gov NCT00141206 NCT00143364.
© 2010 The Authors, Addiction © 2010 Society for the Study of Addiction.
Figures


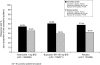

References
-
- Fiore M, Jaén C, Baker T, Benowitz N, Curry S, Dorfman S, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: US Department of Health and Human Services; Public Health Service; 2008.
-
- West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction. 2005;100:299–303. - PubMed
-
- Shiffman S, Sweeney CT, Ferguson SG, Sembower MA, Gitchell JG. Relationship between adherence to daily nicotine patch use and treatment efficacy: secondary analysis of a 10-week randomized, double-blind, placebo-controlled clinical trial simulating over-the-counter use in adult smokers. Clin Ther. 2008;30:1852–8. - PubMed
-
- Lam TH, Abdullah AS, Chan SS, Hedley AJ. Adherence to nicotine replacement therapy versus quitting smoking among Chinese smokers: a preliminary investigation. Psychopharmacology. 2005;177:400–8. - PubMed
-
- Burns EK, Levinson AH. Discontinuation of nicotine replacement therapy among smoking-cessation attempters. Am J Prev Med. 2008;34:212–5. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

