Fusion between human mesenchymal stem cells and rodent cerebellar Purkinje cells
- PMID: 20819172
- PMCID: PMC4150530
- DOI: 10.1111/j.1365-2990.2010.01122.x
Fusion between human mesenchymal stem cells and rodent cerebellar Purkinje cells
Abstract
Aims: we explored whether cellular fusion and heterokaryon formation between human and rodent cells in the cerebellum of mice occurs after intravenous injection of human bone marrow-derived mesenchymal stem cells (MSCs). The influence of central nervous system inflammation on this process was also assessed. In addition, we examined whether tumour necrosis factor (TNF)-alpha and interferon (IFN)-gamma, factors associated with inflammation, increase cellular fusion between human MSCs and rodent cerebellar neurons in vitro.
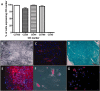
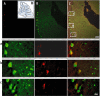
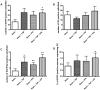
Methods and results: human MSCs were intravenously injected into mice with experimental autoimmune encephalomyelitis (EAE) and control mice. After 22 days, mouse Purkinje cells expressing human Golgi Zone were found within the Purkinje cell layer of the cerebellum, indicating that fusion and heterokaryon formation had occurred. The numbers of heterokaryons in the cerebellum were markedly increased in mice with EAE compared with control mice. Rodent cerebellar neuronal cells labelled with enhanced green fluorescent proteinin vitro were co-cultured with human bone marrow-derived MSCs in the presence of TNF-alpha and/or IFN-gamma to determine their influence on fusion events. We found that fusion between MSCs and cerebellar neurons did occur in vitro and that the frequency of cellular fusion increased in the presence of TNF-alpha and/or IFN-gamma.
Conclusions: we believe that this is the first paper to define fusion and heterokaryon formation between human MSCs and rodent cerebellar neurons in vivo. We have also demonstrated that fusion between these cell populations occurs in vitro. These findings indicate that MSCs may be potential therapeutic agents for cerebellar diseases, and other neuroinflammatory and neurodegenerative disorders.
Figures



Similar articles
-
Cell fusion in the brain: two cells forward, one cell back.Acta Neuropathol. 2014 Nov;128(5):629-38. doi: 10.1007/s00401-014-1303-1. Epub 2014 Jun 5. Acta Neuropathol. 2014. PMID: 24899142 Free PMC article. Review.
-
Aberrant cerebellar Purkinje cell function repaired in vivo by fusion with infiltrating bone marrow-derived cells.Acta Neuropathol. 2018 Jun;135(6):907-921. doi: 10.1007/s00401-018-1833-z. Epub 2018 Mar 14. Acta Neuropathol. 2018. PMID: 29541917 Free PMC article.
-
Effective combination of human bone marrow mesenchymal stem cells and minocycline in experimental autoimmune encephalomyelitis mice.Stem Cell Res Ther. 2013 Jul 5;4(4):77. doi: 10.1186/scrt228. Stem Cell Res Ther. 2013. PMID: 23826999 Free PMC article.
-
Neuroprotection and immunomodulation with mesenchymal stem cells in chronic experimental autoimmune encephalomyelitis.Arch Neurol. 2008 Jun;65(6):753-61. doi: 10.1001/archneur.65.6.753. Arch Neurol. 2008. PMID: 18541795
-
Mesenchymal stem cells as a treatment for multiple sclerosis: a focus on experimental animal studies.Rev Neurosci. 2020 Jan 28;31(2):161-179. doi: 10.1515/revneuro-2019-0040. Rev Neurosci. 2020. PMID: 31605598 Review.
Cited by
-
Cell fusion in the brain: two cells forward, one cell back.Acta Neuropathol. 2014 Nov;128(5):629-38. doi: 10.1007/s00401-014-1303-1. Epub 2014 Jun 5. Acta Neuropathol. 2014. PMID: 24899142 Free PMC article. Review.
-
Bone marrow transplantation stimulates neural repair in Friedreich's ataxia mice.Ann Neurol. 2018 Apr;83(4):779-793. doi: 10.1002/ana.25207. Ann Neurol. 2018. PMID: 29534309 Free PMC article.
-
Cell adhesion and long-term survival of transplanted mesenchymal stem cells: a prerequisite for cell therapy.Oxid Med Cell Longev. 2015;2015:632902. doi: 10.1155/2015/632902. Epub 2015 Feb 2. Oxid Med Cell Longev. 2015. PMID: 25722795 Free PMC article. Review.
-
Stem cell models of polyglutamine diseases and their use in cell-based therapies.Front Neurosci. 2015 Jul 14;9:247. doi: 10.3389/fnins.2015.00247. eCollection 2015. Front Neurosci. 2015. PMID: 26236184 Free PMC article.
-
Mesenchymal stem cells restore frataxin expression and increase hydrogen peroxide scavenging enzymes in Friedreich ataxia fibroblasts.PLoS One. 2011;6(10):e26098. doi: 10.1371/journal.pone.0026098. Epub 2011 Oct 7. PLoS One. 2011. PMID: 22016819 Free PMC article.
References
-
- Himes BT, Neuhuber B, Coleman C, Kushner R, Swanger SA, Kopen GC, Wagner J, Shumsky JS, Fischer I. Recovery of function following grafting of human bone marrow-derived stromal cells into the injured spinal cord. Neurorehabil Neural Repair. 2006;20:278–96. - PubMed
-
- Zhang J, Li Y, Chen J, Yang M, Katakowski M, Lu M, Chopp M. Expression of insulin-like growth factor 1 and receptor in ischemic rats treated with human marrow stromal cells. Brain Res. 2004;1030:19–27. - PubMed
-
- Zhang J, Li Y, Lu M, Cui Y, Chen J, Noffsinger L, Elias SB, Chopp M. Bone marrow stromal cells reduce axonal loss in experimental autoimmune encephalomyelitis mice. J Neurosci Res. 2006;84:587–95. - PubMed
-
- Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–82. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

