Increased expression and local accumulation of the prion protein, Alzheimer Aβ peptides, superoxide dismutase 1, and nitric oxide synthases 1 & 2 in muscle in a rabbit model of diabetes
- PMID: 20819221
- PMCID: PMC2944213
- DOI: 10.1186/1472-6793-10-18
Increased expression and local accumulation of the prion protein, Alzheimer Aβ peptides, superoxide dismutase 1, and nitric oxide synthases 1 & 2 in muscle in a rabbit model of diabetes
Abstract
Background: Muscle disease associated with different etiologies has been shown to produce localized accumulations of amyloid and oxidative stress-related proteins that are more commonly associated with neurodegeneration in the brain. In this study we examined changes in muscle tissue in a classic model of diabetes and hyperglycemia in rabbits to determine if similar dysregulation of Alzheimer Aβ peptides, the prion protein (PrP), and superoxide dismutase 1 (SOD1), as well as nitric oxide synthases is produced in muscle in diabetic animals. This wild-type rabbit model includes systemic physiological expression of human-like Alzheimer precursor proteins and Aβ peptides that are considered key in Alzheimer protein studies.
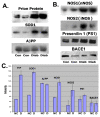
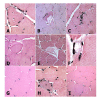
Results: Diabetes was produced in rabbits by injection of the toxic glucose analogue alloxan, which selectively enters pancreatic beta cells and irreversibly decreases insulin production, similar to streptozotocin. Quadriceps muscle from rabbits 16 wks after onset of diabetes and hyperglycemia were analyzed with biochemical and in situ methods. Immunoblots of whole muscle protein samples demonstrated increased PrP, SOD1, as well as neuronal and inducible Nitric oxide synthases (NOS1 and NOS2) in diabetic muscle. In contrast, we detected little change in Alzheimer Aβ precursor protein expression, or BACE1 and Presenilin 1 levels. However, Aβ peptides measured by ELISA increased several fold in diabetic muscle, suggesting a key role for Aβ cleavage in muscle similar to Alzheimer neurodegeneration in this diabetes model. Histological changes in diabetic muscle included localized accumulations of PrP, Aβ, NOS1 and 2, and SOD1, and evidence of increased central nuclei and cell infiltration.
Conclusions: The present study provides evidence that several classic amyloid and oxidative stress-related disease proteins coordinately increase in overall expression and form localized accumulations in diabetic muscle. The present study highlights the capacity of this wild-type animal model to produce an array of hallmark pathological features that have also been described in other muscle diseases.
Figures


References
-
- Barcelo A, Rajpathak S. Incidence and prevalence of diabetes mellitus in the Americas. Rev Panam Salud Publica. 2001;10(5):300–308. - PubMed
-
- Idiculla J, Shirazi N, Opacka-Juffry J, Ganapathi. Diabetic amyotrophy: a brief review. Natl Med J India. 2004;17(4):200–202. - PubMed
-
- Chen X GO, Geiger JD. Rabbits fed cholesterol-enriched diets exhibit pathological features of inclusion body myositis. Am J Physiol Regul Integr Comp Physiol. 2008;294:R829–835. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

