Detecting microcalcifications in atherosclerotic plaques by a simple trichromic staining method for epoxy embedded carotid endarterectomies
- PMID: 20819772
- PMCID: PMC3167311
- DOI: 10.4081/ejh.2010.e33
Detecting microcalcifications in atherosclerotic plaques by a simple trichromic staining method for epoxy embedded carotid endarterectomies
Abstract
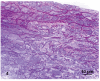
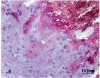
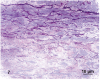
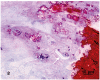
Atherosclerotic plaques have a high probability of undergoing rapid progression to stenosis, becoming responsible of acute coronary syndrome or stroke. Microcalcifications may act as enhancers of atherosclerotic plaque vulnerability. Considering that calcifications with a diameter smaller than 10 mm in paraffin embedded tissue are rather difficult to detect, our aim was to analyze microcalcifications on semithin sections from epoxy resin embedded samples of carotid endarterectomies using an original trichromic stain (methylene blue--azur B--basic fuchsine--alizarin red). We have compared samples stained either with our method, methylene blue-azur B alone or with Von Kossa staining, and methylene blue-azur B -basic fuchsine alone or with Von Kossa staining. Our method resulted to be simple and fast (ca. 2 min), it gives a sharp general contrast for all structures and allows to easy identify collagen and elastin. In addition, gray-green colour associated to intracellular lipid droplets evidences foam cells, which are particularly abundant in endarterectomies samples. Mast cells and their metachromatic granules are also well recognized. Calcifications over 0,5 mm are clearly recognizable. In conclusion, microcalcifications are clearly distinguished from the extracellular matrix in spite of their reduced dimensions. Methylene blue--azur B--basic fuchsine--alizarin red method is easy to use, reproducible, and is particularly suitable for the identification of microcalcifications in the morphological analysis of atherosclerotic plaques.
Figures





Similar articles
-
Small entities with large impact: microcalcifications and atherosclerotic plaque vulnerability.Curr Opin Lipidol. 2014 Oct;25(5):327-32. doi: 10.1097/MOL.0000000000000105. Curr Opin Lipidol. 2014. PMID: 25188916 Free PMC article. Review.
-
A polychromatic staining method for epoxy embedded tissue: a new combination of methylene blue and basic fuchsine for light microscopy.Biotech Histochem. 2005 Sep-Dec;80(5-6):207-10. doi: 10.1080/10520290600560897. Biotech Histochem. 2005. PMID: 16720521
-
An adaptation of Twort's method for polychromatic staining of epoxy-embedded semithin sections.Histochem Cell Biol. 2020 Feb;153(2):121-127. doi: 10.1007/s00418-019-01836-x. Epub 2019 Dec 17. Histochem Cell Biol. 2020. PMID: 31848702
-
[Staining of semithin Durcupan sections].Cesk Patol. 1980 Aug;16(3):158-61. Cesk Patol. 1980. PMID: 6158377 Czech.
-
Calcium deposition within coronary atherosclerotic lesion: Implications for plaque stability.Atherosclerosis. 2020 Aug;306:85-95. doi: 10.1016/j.atherosclerosis.2020.05.017. Epub 2020 Jun 14. Atherosclerosis. 2020. PMID: 32654790 Review.
Cited by
-
3D-Arterial analysis software and CEUS in the assessment of severity and vulnerability of carotid atherosclerotic plaque: a comparison with CTA and histopathology.Radiol Med. 2022 Nov;127(11):1254-1269. doi: 10.1007/s11547-022-01551-z. Epub 2022 Sep 17. Radiol Med. 2022. PMID: 36114929 Free PMC article.
-
Enhancing Lettuce (Lactuca sativa) Productivity: Foliar Sprayed Fe-Alg-CaCO3 MPs as Fertilizers for Aquaponics Cultivation.Plants (Basel). 2024 Dec 5;13(23):3416. doi: 10.3390/plants13233416. Plants (Basel). 2024. PMID: 39683209 Free PMC article.
-
Biocompatibility and Antibiofilm Properties of Calcium Silicate-Based Cements: An In Vitro Evaluation and Report of Two Clinical Cases.Biology (Basel). 2021 May 26;10(6):470. doi: 10.3390/biology10060470. Biology (Basel). 2021. PMID: 34073519 Free PMC article.
-
Small entities with large impact: microcalcifications and atherosclerotic plaque vulnerability.Curr Opin Lipidol. 2014 Oct;25(5):327-32. doi: 10.1097/MOL.0000000000000105. Curr Opin Lipidol. 2014. PMID: 25188916 Free PMC article. Review.
-
On the future contents of a small journal of histochemistry.Eur J Histochem. 2012 Dec 10;56(4):e51. doi: 10.4081/ejh.2012.e51. Eur J Histochem. 2012. PMID: 23361247 Free PMC article.
References
-
- Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108:1664–72. - PubMed
-
- Bluestein D, Alemu Y, Avrahami I, Gharib M, Dumont K, Ricotta JJ, et al. Influence of microcalcifications on vulnerable plaque mechanics using FSI modeling. J Biomech. 2008;41:1111–8. - PubMed
-
- Vengrenyuk Y, Cardoso L, Weinbaum S. Micro-CT based analysis of a new paradigm for vulnerable plaque rupture: cellular microcalcifications in fibrous caps. Mol Cell Biomech. 2008;5:37–47. - PubMed
-
- Jeziorska M, McCollum C, Woolley DE. Calcification in atherosclerotic plaque of human carotid arteries: associations with mast cells and macrophages. J Pathol. 1998;185:10–7. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

