Inflammatory dendritic cells--not basophils--are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen
- PMID: 20819925
- PMCID: PMC2947072
- DOI: 10.1084/jem.20101563
Inflammatory dendritic cells--not basophils--are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen
Abstract
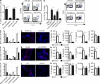
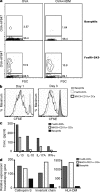
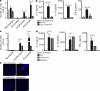
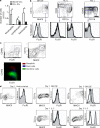
It is unclear how Th2 immunity is induced in response to allergens like house dust mite (HDM). Here, we show that HDM inhalation leads to the TLR4/MyD88-dependent recruitment of IL-4 competent basophils and eosinophils, and of inflammatory DCs to the draining mediastinal nodes. Depletion of basophils only partially reduced Th2 immunity, and depletion of eosinophils had no effect on the Th2 response. Basophils did not take up inhaled antigen, present it to T cells, or express antigen presentation machinery, whereas a population of FceRI(+) DCs readily did. Inflammatory DCs were necessary and sufficient for induction of Th2 immunity and features of asthma, whereas basophils were not required. We favor a model whereby DCs initiate and basophils amplify Th2 immunity to HDM allergen.
Figures


References
-
- Baumeister T., Rössner S., Pech G., de Bruijn M.F., Leenen P.J., Schuler G., Lutz M.B. 2003. Interleukin-3Ralpha+ myeloid dendritic cells and mast cells develop simultaneously from different bone marrow precursors in cultures with interleukin-3. J. Invest. Dermatol. 121:280–288 10.1046/j.1523-1747.2003.12380.x - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

