Protein homeostasis and aging in neurodegeneration
- PMID: 20819932
- PMCID: PMC2935559
- DOI: 10.1083/jcb.201005144
Protein homeostasis and aging in neurodegeneration
Abstract
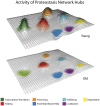
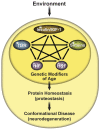
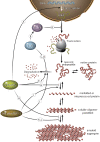
Genetic and environmental factors responsible for numerous neurodegenerative diseases vary between disorders, yet age remains a universal risk factor. Age-associated decline in protein homeostasis, or proteostasis, enables disease-linked proteins to adopt aberrant tertiary structures, accumulate as higher-ordered aggregates, and cause a myriad of cellular dysfunctions and neuronal death. However, recent findings suggest that the assembly of disease proteins into tightly ordered aggregates can significantly delay proteotoxic onset. Furthermore, manipulation of metabolic pathways through key signaling components extends lifespan, bolsters proteostasis networks, and delays the onset of proteotoxicity. Thus, understanding the relationship between proteostasis and aging has provided important insights into neurodegeneration.
Figures
References
-
- Alvira D., Yeste-Velasco M., Folch J., Verdaguer E., Canudas A.M., Pallàs M., Camins A. 2007. Comparative analysis of the effects of resveratrol in two apoptotic models: inhibition of complex I and potassium deprivation in cerebellar neurons. Neuroscience. 147:746–756 10.1016/j.neuroscience.2007.04.029 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

