Mechanistic principles of RAF kinase signaling
- PMID: 20820846
- PMCID: PMC11114552
- DOI: 10.1007/s00018-010-0520-6
Mechanistic principles of RAF kinase signaling
Abstract
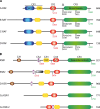
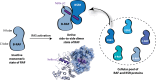
The RAF family of kinases are key components acting downstream of receptor tyrosine kinases and cells employ several distinct mechanisms to strictly control their activity. RAF transitions from an inactive state, where the N-terminal regulatory region binds intramolecularly to the C-terminal kinase domain, to an open state capable of executing the phosphoryl transfer reaction. This transition involves changes both within and between the protein domains in RAF. Many different proteins regulate the transition between inactive and active states of RAF, including RAS and KSR, which are arguably the two most prominent regulators of RAF function. Recent developments have added several new twists to our understanding of RAF regulation. Among others, dimerization of the RAF kinase domain is emerging as a crucial step in the RAF activation process. The multitude of regulatory protein-protein interactions involving RAF remains a largely untapped area for therapeutic applications.
Figures
References
-
- Huebner K, ar-Rushdi A, Griffin CA, Isobe M, Kozak C, Emanuel BS, Nagarajan L, Cleveland JL, Bonner TI, Goldsborough MD, Croce CM, Rapp U. Actively transcribed genes in the raf oncogene group, located on the X chromosome in mouse and human. Proc Natl Acad Sci USA. 1986;83:3934–3938. doi: 10.1073/pnas.83.11.3934. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

