Influenza enhances susceptibility to natural acquisition of and disease due to Streptococcus pneumoniae in ferrets
- PMID: 20822454
- PMCID: PMC3249639
- DOI: 10.1086/656333
Influenza enhances susceptibility to natural acquisition of and disease due to Streptococcus pneumoniae in ferrets
Abstract
The role of respiratory viruses in the transmission of Streptococcus pneumoniae is poorly understood. Key questions, such as which serotypes are most fit for transmission and disease and whether influenza virus alters these parameters in a serotype-specific manner, have not been adequately studied. In a novel model of transmission in ferrets, we demonstrated that pneumococcal transmission and disease were enhanced if donors had previously been infected with influenza virus. Bacterial titers in nasal wash, the incidence of mucosal and invasive disease, and the percentage of contacts that were infected all increased. In contact ferrets, viral infection increased their susceptibility to S. pneumoniae acquisition both in terms of the percentage infected and the distance over which they could acquire infection. These influenza-mediated effects on colonization, transmission, and disease were dependent on the pneumococcal strain. Overall, these data argue that the relationship between respiratory viral infections, acquisition of pneumococci, and development of disease in humans needs further study to be better understood.
Conflict of interest statement
The authors declare no conflicts of interest exist.
Figures

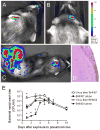
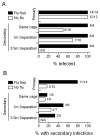


Comment in
-
Sequential infections with influenza and novel respiratory bacteria.J Infect Dis. 2011 Apr 1;203(7):1034-5. doi: 10.1093/infdis/jiq149. J Infect Dis. 2011. PMID: 21402556 No abstract available.
-
Unveiling the burden of influenza-associated pneumococcal pneumonia.J Infect Dis. 2012 Feb 1;205(3):355-7. doi: 10.1093/infdis/jir753. Epub 2011 Dec 7. J Infect Dis. 2012. PMID: 22158562 Free PMC article. No abstract available.
References
-
- Bridy-Pappas AE, Margolis MB, Center KJ, Isaacman DJ. Streptococcus pneumoniae: description of the pathogen, disease epidemiology, treatment, and prevention. Pharmacotherapy. 2005;25(9):1193–212. - PubMed
-
- De Wals P, Erickson L, Poirier B, Pepin J, Pichichero ME. How to compare the efficacy of conjugate vaccines to prevent acute otitis media? Vaccine. 2009;27(21):2877–83. - PubMed
-
- Peltola VT, McCullers JA. Respiratory viruses predisposing to bacterial infections: role of neuraminidase. Pediatr Infect Dis J. 2004;23(1 Suppl):S87–S97. - PubMed
-
- Finland M. Recent advances in the epidemiology of pneumococcal infections. Medicine (Baltimore) 1942;21(3):307–44.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

