Rac and Rho GTPases in cancer cell motility control
- PMID: 20822528
- PMCID: PMC2941746
- DOI: 10.1186/1478-811X-8-23
Rac and Rho GTPases in cancer cell motility control
Abstract
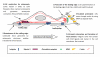
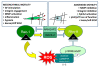
Rho GTPases represent a family of small GTP-binding proteins involved in cell cytoskeleton organization, migration, transcription, and proliferation. A common theme of these processes is a dynamic reorganization of actin cytoskeleton which has now emerged as a major switch control mainly carried out by Rho and Rac GTPase subfamilies, playing an acknowledged role in adaptation of cell motility to the microenvironment. Cells exhibit three distinct modes of migration when invading the 3 D environment. Collective motility leads to movement of cohorts of cells which maintain the adherens junctions and move by photolytic degradation of matrix barriers. Single cell mesenchymal-type movement is characterized by an elongated cellular shape and again requires extracellular proteolysis and integrin engagement. In addition it depends on Rac1-mediated cell polarization and lamellipodia formation. Conversely, in amoeboid movement cells have a rounded morphology, the movement is independent from proteases but requires high Rho GTPase to drive elevated levels of actomyosin contractility. These two modes of cell movement are interconvertible and several moving cells, including tumor cells, show an high degree of plasticity in motility styles shifting ad hoc between mesenchymal or amoeboid movements. This review will focus on the role of Rac and Rho small GTPases in cell motility and in the complex relationship driving the reciprocal control between Rac and Rho granting for the opportunistic motile behaviour of aggressive cancer cells. In addition we analyse the role of these GTPases in cancer progression and metastatic dissemination.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

