Inhibition of class II histone deacetylases in the spinal cord attenuates inflammatory hyperalgesia
- PMID: 20822541
- PMCID: PMC2942827
- DOI: 10.1186/1744-8069-6-51
Inhibition of class II histone deacetylases in the spinal cord attenuates inflammatory hyperalgesia
Abstract
Background: Several classes of histone deacetylases (HDACs) are expressed in the spinal cord that is a critical structure of the nociceptive pathway. HDAC-regulated histone acetylation is an important component of chromatin remodeling leading to epigenetic regulation of gene transcription. To understand the role of histone acetylation in epigenetic regulation of pathological pain, we have studied the impact of different classes of HDACs in the spinal cord on inflammatory hyperalgesia induced by complete Freund's adjuvant (CFA).
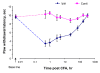
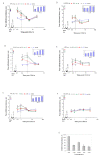
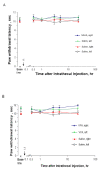
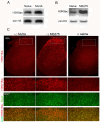
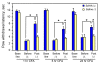
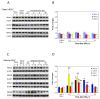
Results: We intrathecally applied inhibitors specific to different classes of HDACs and evaluated their impact on inflammatory hyperalgesia. Pre-injected inhibitors targeting class I as well as II (SAHA, TSA, LAQ824) or IIa (VPA, 4-PB) HDACs significantly delayed the thermal hyperalgesia induced by unilateral CFA injection in the hindpaw. Existing hyperalgesia induced by CFA was also attenuated by the HDAC inhibitors (HDACIs). In contrast, these inhibitors did not interfere with the thermal response either in naïve animals, or on the contralateral side of inflamed animals. Interestingly, MS-275 that specifically inhibits class I HDACs failed to alter the hyperalgesia although it increased histone 3 acetylation in the spinal cord as SAHA did. Using immunoblot analysis, we further found that the levels of class IIa HDAC members (HDAC4, 5, 7, 9) in the spinal dorsal horn were upregulated following CFA injection while those of class I HDAC members (HDAC1, 2, 3) remained stable or were slightly reduced.
Conclusions: Our data suggest that activity of class II HDACs in the spinal cord is critical to the induction and maintenance of inflammatory hyperalgesia induced by CFA, while activity of class I HDACs may be unnecessary. Comparison of the effects of HDACIs specific to class II and IIa as well as the expression pattern of different HDACs in the spinal cord in response to CFA suggests that the members of class IIa HDACs may be potential targets for attenuating persistent inflammatory pain.
Figures
References
-
- Geranton SM, Morenilla-Palao C, Hunt SP. A role for transcriptional repressor methyl-CpG-binding protein 2 and plasticity-related gene serum- and glucocorticoid-inducible kinase 1 in the induction of inflammatory pain states. J Neurosci. 2007;27:6163–6173. doi: 10.1523/JNEUROSCI.1306-07.2007. - DOI - PMC - PubMed
-
- Cheng HY, Pitcher GM, Laviolette SR, Whishaw IQ, Tong KI, Kockeritz LK, Wada T, Joza NA, Crackower M, Goncalves J, Sarosi I, Woodgett JR, Oliveira-dos-Santos AJ, Ikura M, van der Kooy D, Salter MW, Penninger JM. DREAM is a critical transcriptional repressor for pain modulation. Cell. 2002;108:31–43. doi: 10.1016/S0092-8674(01)00629-8. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

