Relationship of dementia screening tests with biomarkers of Alzheimer's disease
- PMID: 20823087
- PMCID: PMC2965421
- DOI: 10.1093/brain/awq204
Relationship of dementia screening tests with biomarkers of Alzheimer's disease
Abstract
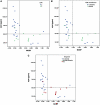
Screening tests for Alzheimer's disease lack sensitivity and specificity. We developed the AD8, a brief dementia screening interview validated against clinical and cognitive evaluations, as an improvement over current screening methods. Because insufficient follow-up has occurred to validate the AD8 against the neuropathologic findings of Alzheimer's disease, we investigated whether AD8 scores correspond to impairment in episodic memory testing and changes in biomarkers of Alzheimer's disease (cerebrospinal fluid and amyloid imaging with Pittsburgh compound B) characteristic of symptomatic Alzheimer's disease. We also compared informant-based assessments with brief performance-based dementia screening measurements such as the Mini Mental State Exam. The sample (n = 257) had a mean age of 75.4 years with 15.1 years of education; 88.7% were Caucasian and 45.5% were male. The sample was divided into two groups based on their AD8 scores: those with a negative dementia screening test (AD8 score 0 or 1, n = 137) and those with a positive dementia screening test (AD8 score ≥2, n = 120). Individuals with positive AD8 scores had abnormal Pittsburgh compound B binding (P < 0.001) and cerebrospinal fluid biomarkers (P < 0.001) compared with individuals with negative AD8 scores. Individuals with positive AD8 tests and positive biomarkers scored in the impaired range on the Wechsler Logical Memory Story A (mean score 7.0 ± 4.5 for Pittsburgh compound B; mean score 7.6 ± 5.3 for cerebrospinal fluid amyloid beta protein 1-42). The AD8 area under the curve for Pittsburgh compound B was 0.737 (95% confidence interval: 0.64-0.83) and for cerebrospinal fluid amyloid beta protein 1-42 was 0.685 (95% confidence interval: 0.60-0.77) suggesting good discrimination. The AD8 had superior sensitivity in detecting early stages of dementia compared with the Mini Mental State Examination. The AD8 had a likelihood ratio of a positive test of 5.8 (95% confidence interval: 5.4-6.3) and likelihood ratio of a negative test of 0.04 (95% confidence interval: 0.03-0.06), increasing the pre-test probability of an individual having symptomatic Alzheimer's disease. Individuals with AD8 scores of ≥2 had a biomarker phenotype consistent with Alzheimer's disease and lower performance on episodic memory tests, supporting a diagnosis of Alzheimer's disease. Informant-based assessments may be superior to performance-based screening measures such as the Mini Mental State Examination in corresponding to underlying Alzheimer's disease pathology, particularly at the earliest stages of decline. The use of a brief test such as the AD8 may improve strategies for detecting dementia in community settings where biomarkers may not be readily available, and may enrich clinical trial recruitment by increasing the likelihood that participants have underlying biomarker abnormalities.
Figures
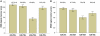
Comment in
-
The impact of dementia prevalence on the utility of the AD8.Brain. 2012 Jan;135(Pt 1):e203; author reply e204. doi: 10.1093/brain/awr135. Epub 2011 Jun 8. Brain. 2012. PMID: 21653541 Free PMC article. No abstract available.
References
-
- American Academy of Neurology. http://www.aan.com/professionals/practice/pdfs/dementia_guideline.pdf (3 January 2010, date last accessed)
-
- American Geriatrics Society. http://www.americangeriatrics.org/products/positionpapers/stopscreening.... (3 January 2010, date last accessed)
-
- American Medical Association. Practical guide for the Primary Care Physician on the Diagnosis, Management and Treatment of Dementia. Program on Aging and Community Health. Chicago, IL, 2001. Available at: http://www.ama-assn.org/ama/pub/category/4789.html (3 January 2010, date last accessed)
-
- Armitage SG. An analysis of certain psychological tests used in the evaluation of brain injury. Psych Mono. 1946;60:1–48.
-
- Benton AL. The Revised Visual Retention Test: clinical and experimental applications. New York: Psychological Corporation; 1963.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

