Antiatherosclerotic and renoprotective effects of ebselen in the diabetic apolipoprotein E/GPx1-double knockout mouse
- PMID: 20823099
- PMCID: PMC2992783
- DOI: 10.2337/db10-0195
Antiatherosclerotic and renoprotective effects of ebselen in the diabetic apolipoprotein E/GPx1-double knockout mouse
Abstract
Objective: To investigate the effect of the GPx1-mimetic ebselen on diabetes-associated atherosclerosis and renal injury in a model of increased oxidative stress.
Research design and methods: The study was performed using diabetic apolipoprotein E/GPx1 (ApoE(-/-)GPx1(-/-))-double knockout (dKO) mice, a model combining hyperlipidemia and hyperglycemia with increased oxidative stress. Mice were randomized into two groups, one injected with streptozotocin, the other with vehicle, at 8 weeks of age. Groups were further randomized to receive either ebselen or no treatment for 20 weeks.
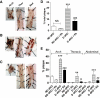
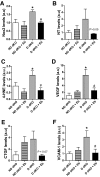
Results: Ebselen reduced diabetes-associated atherosclerosis in most aortic regions, with the exception of the aortic sinus, and protected dKO mice from renal structural and functional injury. The protective effects of ebselen were associated with a reduction in oxidative stress (hydroperoxides in plasma, 8-isoprostane in urine, nitrotyrosine in the kidney, and 4-hydroxynonenal in the aorta) as well as a reduction in VEGF, CTGF, VCAM-1, MCP-1, and Nox2 after 10 weeks of diabetes in the dKO aorta. Ebselen also significantly reduced the expression of proteins implicated in fibrosis and inflammation in the kidney as well as reducing related key intracellular signaling pathways.
Conclusions: Ebselen has an antiatherosclerotic and renoprotective effect in a model of accelerated diabetic complications in the setting of enhanced oxidative stress. Our data suggest that ebselen effectively repletes the lack of GPx1, and indicate that ebselen may be an effective therapeutic for the treatment of diabetes-related atherosclerosis and nephropathy. Furthermore, this study highlights the feasibility of addressing two diabetic complications with one treatment regimen through the unifying approach of targeted antioxidant therapy.
Figures






References
-
- Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW: Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003;108:2154–2169 - PubMed
-
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M: Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229–234 - PubMed
-
- Schiele F: Renal dysfunction and coronary disease: a high-risk combination. J Nephrol 2009;22:39–45 - PubMed
-
- Okamura DM, Himmelfarb J: Tipping the redox balance of oxidative stress in fibrogenic pathways in chronic kidney disease. Pediatr Nephrol 2009;24:2309–2319 - PubMed
-
- Da Ros R, Assaloni R, Ceriello A: Antioxidant therapy in diabetic complications: what is new? Curr Vasc Pharmacol 2004;2:335–341 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

