Mannheimia haemolytica and its leukotoxin cause neutrophil extracellular trap formation by bovine neutrophils
- PMID: 20823211
- PMCID: PMC2976348
- DOI: 10.1128/IAI.00840-10
Mannheimia haemolytica and its leukotoxin cause neutrophil extracellular trap formation by bovine neutrophils
Abstract
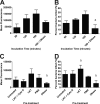
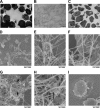
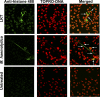
Mannheimia haemolytica is an important member of the bovine respiratory disease complex, which is characterized by abundant neutrophil infiltration into the alveoli and fibrin deposition. Recently several authors have reported that human neutrophils release neutrophil extracellular traps (NETs), which are protein-studded DNA matrices capable of trapping and killing pathogens. Here, we demonstrate that the leukotoxin (LKT) of M. haemolytica causes NET formation by bovine neutrophils in a CD18-dependent manner. Using an unacylated, noncytotoxic pro-LKT produced by an ΔlktC mutant of M. haemolytica, we show that binding of unacylated pro-LKT stimulates NET formation despite a lack of cytotoxicity. Inhibition of LKT binding to the CD18 chain of lymphocyte function-associated antigen 1 (LFA-1) on bovine neutrophils reduced NET formation in response to LKT or M. haemolytica cells. Further investigation revealed that NETs formed in response to M. haemolytica are capable of trapping and killing a portion of the bacterial cells. NET formation was confirmed by confocal microscopy and by scanning and transmission electron microscopy. Prior exposure of bovine neutrophils to LKT enhanced subsequent trapping and killing of M. haemolytica cells in bovine NETs. Understanding NET formation in response to M. haemolytica and its LKT provides a new perspective on how neutrophils contribute to the pathogenesis of bovine respiratory disease.
Figures


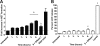


References
-
- Ackermann, M. R., B. M. DeBey, T. J. Stabel, J. H. Gold, K. B. Register, and J. T. Meehan. 1994. Distribution of anti-CD68 (EBM11) immunoreactivity in formalin-fixed, paraffin-embedded bovine tissues. Vet. Pathol. 31:340-348. - PubMed
-
- Alexander, L. E., H. C. Maisey, A. M. Timmer, S. H. Rooijakkers, R. L. Gallo, M. von Kockritz-Blickwede, and V. Nizet. 2010. M1T1 group A streptococcal pili promote epithelial colonization but diminish systemic virulence through neutrophil extracellular entrapment. J. Mol. Med. 88:371-381. - PMC - PubMed
-
- Ambagala, T. C., A. P. Ambagala, and S. Srikumaran. 1999. The leukotoxin of Pasteurella haemolytica binds to beta(2) integrins on bovine leukocytes. FEMS Microbol. Lett. 179:161-167. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

