Targeting botulinum neurotoxin persistence by the ubiquitin-proteasome system
- PMID: 20823219
- PMCID: PMC2944746
- DOI: 10.1073/pnas.1008302107
Targeting botulinum neurotoxin persistence by the ubiquitin-proteasome system
Abstract
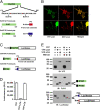
Botulinum neurotoxins (BoNTs) are the most potent natural toxins known. The effects of BoNT serotype A (BoNT/A) can last several months, whereas the effects of BoNT serotype E (BoNT/E), which shares the same synaptic target, synaptosomal-associated protein 25 (SNAP25), last only several weeks. The long-lasting effects or persistence of BoNT/A, although desirable for therapeutic applications, presents a challenge for medical treatment of BoNT intoxication. Although the mechanisms for BoNT toxicity are well known, little is known about the mechanisms that govern the persistence of the toxins. We show that the recombinant catalytic light chain (LC) of BoNT/E is ubiquitylated and rapidly degraded in cells. In contrast, BoNT/A LC is considerably more stable. Differential susceptibility of the catalytic LCs to ubiquitin-dependent proteolysis therefore might explain the differential persistence of BoNT serotypes. In this regard we show that TRAF2, a RING finger protein implicated in ubiquitylation, selectively associates with BoNT/E LC and promotes its proteasomal degradation. Given these data, we asked whether BoNT/A LC could be targeted for rapid proteasomal degradation by redirecting it to characterized ubiquitin ligase domains. We describe chimeric SNAP25-based ubiquitin ligases that target BoNT/A LC for degradation, reducing its duration in a cellular model for toxin persistence.
Conflict of interest statement
Conflict of interest statement: G.A.O. has a financial interest in Synaptic Research, LLC, which is developing therapeutics for BoNT intoxication including designs based on ubiquitin ligases.
Figures

References
-
- Simpson LL. Identification of the characteristics that underlie botulinum toxin potency: Implications for designing novel drugs. Biochimie. 2000;82:943–953. - PubMed
-
- Habermann E, Dreyer F. Clostridial neurotoxins: Handling and action at the cellular and molecular level. Curr Top Microbiol Immunol. 1986;129:93–179. - PubMed
-
- Jahn R, Niemann H. Molecular mechanisms of clostridial neurotoxins. Ann N Y Acad Sci. 1994;733:245–255. - PubMed
-
- Montecucco C, Schiavo G. Structure and function of tetanus and botulinum neurotoxins. Q Rev Biophys. 1995;28:423–472. - PubMed
-
- Eleopra R, Tugnoli V, Rossetto O, De Grandis D, Montecucco C. Different time courses of recovery after poisoning with botulinum neurotoxin serotypes A and E in humans. Neurosci Lett. 1998;256:135–138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

