Rigid amphipathic fusion inhibitors, small molecule antiviral compounds against enveloped viruses
- PMID: 20823220
- PMCID: PMC2951442
- DOI: 10.1073/pnas.1010026107
Rigid amphipathic fusion inhibitors, small molecule antiviral compounds against enveloped viruses
Abstract
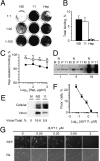
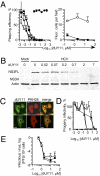
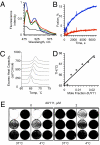
Antiviral drugs targeting viral proteins often result in prompt selection for resistance. Moreover, the number of viral targets is limited. Novel antiviral targets are therefore needed. The unique characteristics of fusion between virion envelopes and cell membranes may provide such targets. Like all fusing bilayers, viral envelopes locally adopt hourglass-shaped stalks during the initial stages of fusion, a process that requires local negative membrane curvature. Unlike cellular vesicles, however, viral envelopes do not redistribute lipids between leaflets, can only use the energy released by virion proteins, and fuse to the extracellular leaflets of cell membranes. Enrichment in phospholipids with hydrophilic heads larger than their hydrophobic tails in the convex outer leaflet of vesicles favors positive curvature, therefore increasing the activation energy barrier for fusion. Such phospholipids can increase the activation barrier beyond the energy provided by virion proteins, thereby inhibiting viral fusion. However, phospholipids are not pharmacologically useful. We show here that a family of synthetic rigid amphiphiles of shape similar to such phospholipids, RAFIs (rigid amphipathic fusion inhibitors), inhibit the infectivity of several otherwise unrelated enveloped viruses, including hepatitis C and HSV-1 and -2 (lowest apparent IC(50) 48 nM), with no cytotoxic or cytostatic effects (selectivity index > 3,000) by inhibiting the increased negative curvature required for the initial stages of fusion.
Conflict of interest statement
Conflict of interest statement: M.R.S., A.V.U., and L.M.S. are co-inventors in a series of patent applications describing these discoveries.
Figures

Comment in
-
Driving a wedge between viral lipids blocks infection.Proc Natl Acad Sci U S A. 2010 Oct 5;107(40):17069-70. doi: 10.1073/pnas.1012748107. Epub 2010 Sep 27. Proc Natl Acad Sci U S A. 2010. PMID: 20876093 Free PMC article. No abstract available.
References
-
- Pillay D. The priorities for antiviral drug resistance surveillance and research. J Antimicrob Chemother. 2007;60(Suppl 1):i57–i58. - PubMed
-
- Phillips AN, et al. UK Collaborative HIV Cohort (CHIC) Study Risk of extensive virological failure to the three original antiretroviral drug classes over long-term follow-up from the start of therapy in patients with HIV infection: An observational cohort study. Lancet. 2007;370:1923–1928. - PubMed
-
- De Clercq E, Brancale A, Hodge AV, Field HJ. Antiviral chemistry and chemotherapy's current antiviral agents FactFile 2006 (1st edition) Antivir Chem Chemother. 2006;17:113–166. - PubMed
-
- De Clercq E. Three decades of antiviral drugs. Nat Rev Drug Discov. 2007;6:941.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

