A small molecule accelerates neuronal differentiation in the adult rat
- PMID: 20823227
- PMCID: PMC2944756
- DOI: 10.1073/pnas.1010300107
A small molecule accelerates neuronal differentiation in the adult rat
Erratum in
- Proc Natl Acad Sci U S A. 2010 Dec 21;107(51):22360. Halder, Rajkumar [added]
Abstract
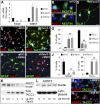
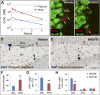
Adult neurogenesis occurs in mammals and provides a mechanism for continuous neural plasticity in the brain. However, little is known about the molecular mechanisms regulating hippocampal neural progenitor cells (NPCs) and whether their fate can be pharmacologically modulated to improve neural plasticity and regeneration. Here, we report the characterization of a small molecule (KHS101) that selectively induces a neuronal differentiation phenotype. Mechanism of action studies revealed a link of KHS101 to cell cycle exit and specific binding to the TACC3 protein, whose knockdown in NPCs recapitulates the KHS101-induced phenotype. Upon systemic administration, KHS101 distributed to the brain and resulted in a significant increase in neuronal differentiation in vivo. Our findings indicate that KHS101 accelerates neuronal differentiation by interaction with TACC3 and may provide a basis for pharmacological intervention directed at endogenous NPCs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. - PubMed
-
- Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: Reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562–1571. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

