Corepressor for element-1-silencing transcription factor preferentially mediates gene networks underlying neural stem cell fate decisions
- PMID: 20823235
- PMCID: PMC2944745
- DOI: 10.1073/pnas.0906917107
Corepressor for element-1-silencing transcription factor preferentially mediates gene networks underlying neural stem cell fate decisions
Abstract
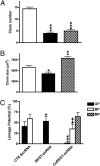
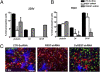
The repressor element-1 (RE1) silencing transcription factor/neuron-restrictive silencer factor (REST/NRSF) silences neuronal genes in neural stem cells (NSCs) and nonneuronal cells through its role as a dynamic modular platform for recruitment of transcriptional and epigenetic regulatory cofactors to RE1-containing promoters. In embryonic stem cells, the REST regulatory network is highly integrated with the transcriptional circuitry governing self-renewal and pluripotency, although its exact functional role is unclear. The C-terminal cofactor for REST, CoREST, also acts as a modular scaffold, but its cell type-specific roles have not been elucidated. We used chromatin immunoprecipitation-on-chip to examine CoREST and REST binding sites in NSCs and their proximate progenitor species. In NSCs, we identified a larger number of CoREST (1,820) compared with REST (322) target genes. The majority of these CoREST targets do not contain known RE1 motifs. Notably, these CoREST target genes do play important roles in pluripotency networks, in modulating NSC identity and fate decisions and in epigenetic processes previously associated with both REST and CoREST. Moreover, we found that NSC-mediated developmental transitions were associated primarily with liberation of CoREST from promoters with transcriptional repression favored in less lineage-restricted radial glia and transcriptional activation favored in more lineage-restricted neuronal-oligodendrocyte precursors. Clonal NSC REST and CoREST gene manipulation paradigms further revealed that CoREST has largely independent and previously uncharacterized roles in promoting NSC multilineage potential and modulating early neural fate decisions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
REST and CoREST modulate neuronal subtype specification, maturation and maintenance.PLoS One. 2009 Dec 7;4(12):e7936. doi: 10.1371/journal.pone.0007936. PLoS One. 2009. PMID: 19997604 Free PMC article.
-
Differential deployment of REST and CoREST promotes glial subtype specification and oligodendrocyte lineage maturation.PLoS One. 2009 Nov 3;4(11):e7665. doi: 10.1371/journal.pone.0007665. PLoS One. 2009. PMID: 19888342 Free PMC article.
-
REST regulates distinct transcriptional networks in embryonic and neural stem cells.PLoS Biol. 2008 Oct 28;6(10):e256. doi: 10.1371/journal.pbio.0060256. PLoS Biol. 2008. PMID: 18959480 Free PMC article.
-
REST and CoREST are transcriptional and epigenetic regulators of seminal neural fate decisions.Cell Cycle. 2010 Nov 15;9(22):4477-86. doi: 10.4161/cc.9.22.13973. Epub 2010 Nov 15. Cell Cycle. 2010. PMID: 21088488 Free PMC article. Review.
-
More than a Corepressor: The Role of CoREST Proteins in Neurodevelopment.eNeuro. 2020 Mar 10;7(2):ENEURO.0337-19.2020. doi: 10.1523/ENEURO.0337-19.2020. Print 2020 Mar/Apr. eNeuro. 2020. PMID: 32075869 Free PMC article. Review.
Cited by
-
Endotype Characterization Reveals Mechanistic Differences Across Brain Regions in Sporadic Alzheimer's Disease.J Alzheimers Dis Rep. 2023 Aug 29;7(1):957-972. doi: 10.3233/ADR-220098. eCollection 2023. J Alzheimers Dis Rep. 2023. PMID: 37849634 Free PMC article.
-
LSD1 co-repressor Rcor2 orchestrates neurogenesis in the developing mouse brain.Nat Commun. 2016 Jan 22;7:10481. doi: 10.1038/ncomms10481. Nat Commun. 2016. PMID: 26795843 Free PMC article.
-
Repressor element-1 silencing transcription factor (REST)-dependent epigenetic remodeling is critical to ischemia-induced neuronal death.Proc Natl Acad Sci U S A. 2012 Apr 17;109(16):E962-71. doi: 10.1073/pnas.1121568109. Epub 2012 Feb 27. Proc Natl Acad Sci U S A. 2012. PMID: 22371606 Free PMC article.
-
ZNF750 interacts with KLF4 and RCOR1, KDM1A, and CTBP1/2 chromatin regulators to repress epidermal progenitor genes and induce differentiation genes.Genes Dev. 2014 Sep 15;28(18):2013-26. doi: 10.1101/gad.246579.114. Genes Dev. 2014. PMID: 25228645 Free PMC article.
-
miR-9 utilizes precursor pathways in adaptation to alcohol in mouse striatal neurons.Adv Drug Alcohol Res. 2023;3:11323. doi: 10.3389/adar.2023.11323. Epub 2023 Jun 6. Adv Drug Alcohol Res. 2023. PMID: 38116240 Free PMC article.
References
-
- Ballas N, Mandel G. The many faces of REST oversee epigenetic programming of neuronal genes. Curr Opin Neurobiol. 2005;15:500–506. - PubMed
-
- Schoenherr CJ, Anderson DJ. The neuron-restrictive silencer factor (NRSF): A coordinate repressor of multiple neuron-specific genes. Science. 1995;267:1360–1363. - PubMed
-
- Chong JA, et al. REST: A mammalian silencer protein that restricts sodium channel gene expression to neurons. Cell. 1995;80:949–957. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

