Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor
- PMID: 20823238
- PMCID: PMC2947883
- DOI: 10.1073/pnas.1012443107
Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor
Abstract
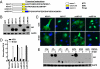
Androgen receptor (AR) splice variants lacking the ligand binding domain (ARVs), originally isolated from prostate cancer cell lines derived from a single patient, are detected in normal and malignant human prostate tissue, with the highest levels observed in late stage, castration-resistant prostate cancer. The most studied variant (called AR-V7 or AR3) activates AR reporter genes in the absence of ligand and therefore, could play a role in castration resistance. To explore the range of potential ARVs, we screened additional human and murine prostate cancer models using conventional and next generation sequencing technologies and detected several structurally diverse AR isoforms. Some, like AR-V7/AR3, display gain of function, whereas others have dominant interfering activity. We also find that ARV expression increases acutely in response to androgen withdrawal, is suppressed by testosterone, and in some models, is coupled to full-length AR (AR-FL) mRNA production. As expected, constitutively active, ligand-independent ARVs such as AR-V7/AR3 are sufficient to confer anchorage-independent (in vitro) and castration-resistant (in vivo) growth. Surprisingly, this growth is blocked by ligand binding domain-targeted antiandrogens, such as MDV3100, or by selective siRNA silencing of AR-FL, indicating that the growth-promoting effects of ARVs are mediated through AR-FL. These data indicate that the increase in ARV expression in castrate-resistant prostate cancer is an acute response to castration rather than clonal expansion of castration or antiandrogen-resistant cells expressing gain of function ARVs, and furthermore, they provide a strategy to overcome ARV function in the clinic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

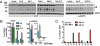



Comment in
-
Prostate cancer: New insight into mechanisms of castration resistance.Nat Rev Urol. 2010 Nov;7(11):590. doi: 10.1038/nrurol.2010.171. Nat Rev Urol. 2010. PMID: 21113989 No abstract available.
References
-
- Lamont KR, Tindall DJ. Androgen regulation of gene expression. Adv Cancer Res. 2010;107:137–162. - PubMed
-
- Brinkmann AO. Molecular basis of androgen insensitivity. Mol Cell Endocrinol. 2001;179:105–109. - PubMed
-
- Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: Directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–8261. - PubMed
-
- Nagabhushan M, et al. CWR22: The first human prostate cancer xenograft with strongly androgen-dependent and relapsed strains both in vivo and in soft agar. Cancer Res. 1996;56:3042–3046. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

