Expression of skeletal muscle sodium channel (Nav1.4) or connexin32 prevents reperfusion arrhythmias in murine heart
- PMID: 20823275
- PMCID: PMC3002874
- DOI: 10.1093/cvr/cvq284
Expression of skeletal muscle sodium channel (Nav1.4) or connexin32 prevents reperfusion arrhythmias in murine heart
Abstract
Aims: acute myocardial ischaemia induces a decrease in resting membrane potential [which leads to reduction of action potential (AP) V(max)] and intracellular acidification (which closes gap junctions). Both contribute to conduction slowing. We hypothesized that ventricular expression of the skeletal muscle Na(+) channel, Nav1.4 (which activates fully at low membrane potentials), or connexin32 (Cx32, which is less pH-sensitive than connexin43) would support conduction and be antiarrhythmic. We tested this hypothesis in a murine model of ischaemia and reperfusion arrhythmias.
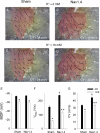
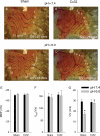
Methods and results: empty adenovirus (Sham) or adenoviral constructs expressing either SkM1 (gene encoding Nav1.4) or Cx32 genes were injected into the left ventricular wall. Four days later, ventricular tachycardia (VT) occurred during reperfusion following a 5 min coronary occlusion. In Nav1.4- and Cx32-expressing mice, VT incidence and duration were lower than in Sham (P < 0.05). In vitro multisite microelectrode mapping was performed in the superfused right ventricular wall. To simulate ischaemic conditions, [K(+)] in solution was increased to 10 mmol/L and/or pH was decreased to 6.0. Western blots revealed Cx32 and Nav1.4 expression in both ventricles. Nav1.4 APs showed higher V(max) and conduction velocity (CV) than Shams at normal and elevated [K(+)]. Exposure of tissue to acid solution reduced intracellular pH to 6.4. There was no difference in CV between Sham and Cx32 groups in control solution. Acid solution slowed CV in Sham (P < 0.05) but not in Cx32.
Conclusion: Nav1.4 or Cx32 expression preserved normal conduction in murine hearts and decreased the incidence of reperfusion VT.
Figures




Comment in
-
Exciting treatment of reentrant arrhythmias.Cardiovasc Res. 2011 Jan 1;89(1):4-5. doi: 10.1093/cvr/cvq347. Epub 2010 Nov 4. Cardiovasc Res. 2011. PMID: 21051418 No abstract available.
Similar articles
-
Epicardial border zone overexpression of skeletal muscle sodium channel SkM1 normalizes activation, preserves conduction, and suppresses ventricular arrhythmia: an in silico, in vivo, in vitro study.Circulation. 2009 Jan 6;119(1):19-27. doi: 10.1161/CIRCULATIONAHA.108.809301. Epub 2008 Dec 22. Circulation. 2009. PMID: 19103989 Free PMC article.
-
Expression of skeletal but not cardiac Na+ channel isoform preserves normal conduction in a depolarized cardiac syncytium.Cardiovasc Res. 2009 Feb 15;81(3):528-35. doi: 10.1093/cvr/cvn290. Epub 2008 Oct 31. Cardiovasc Res. 2009. PMID: 18977767 Free PMC article.
-
SkM1 and Cx32 improve conduction in canine myocardial infarcts yet only SkM1 is antiarrhythmic.Cardiovasc Res. 2012 Jun 1;94(3):450-9. doi: 10.1093/cvr/cvs107. Epub 2012 Feb 27. Cardiovasc Res. 2012. PMID: 22374989 Free PMC article.
-
Ventricular arrhythmogenesis: insights from murine models.Prog Biophys Mol Biol. 2008 Oct-Nov;98(2-3):208-18. doi: 10.1016/j.pbiomolbio.2008.10.011. Epub 2008 Nov 9. Prog Biophys Mol Biol. 2008. PMID: 19041335 Review.
-
Cardiac sodium channel Nav1.5 and its associated proteins.Arch Mal Coeur Vaiss. 2007 Sep;100(9):787-93. Arch Mal Coeur Vaiss. 2007. PMID: 18033008 Review.
Cited by
-
Effects of different LAD-blocked sites on the development of acute myocardial infarction and malignant arrhythmia in a swine model.J Thorac Dis. 2014 Sep;6(9):1271-7. doi: 10.3978/j.issn.2072-1439.2014.07.22. J Thorac Dis. 2014. PMID: 25276369 Free PMC article.
-
SCN10A-short gene therapy to restore conduction and protect against malignant cardiac arrhythmias.Eur Heart J. 2025 May 7;46(18):1747-1762. doi: 10.1093/eurheartj/ehaf053. Eur Heart J. 2025. PMID: 39973098 Free PMC article.
-
Effect of skeletal muscle Na(+) channel delivered via a cell platform on cardiac conduction and arrhythmia induction.Circ Arrhythm Electrophysiol. 2012 Aug 1;5(4):831-40. doi: 10.1161/CIRCEP.111.969907. Epub 2012 Jun 21. Circ Arrhythm Electrophysiol. 2012. PMID: 22722661 Free PMC article.
-
Cell and gene therapy for arrhythmias: Repair of cardiac conduction damage.J Geriatr Cardiol. 2011 Sep;8(3):147-58. doi: 10.3724/SP.J.1263.2011.00147. J Geriatr Cardiol. 2011. PMID: 22783301 Free PMC article.
-
Association of attenuated leptin signaling pathways with impaired cardiac function under prolonged high-altitude hypoxia.Sci Rep. 2024 May 3;14(1):10206. doi: 10.1038/s41598-024-59559-6. Sci Rep. 2024. PMID: 38702334 Free PMC article.
References
-
- Murdock DK, Loeb JM, Euler DE, Randall WC. Electrophysiology of coronary reperfusion: a mechanism for reperfusion arrhythmias. Circulation. 1980;61:175–182. - PubMed
-
- Manning AS, Hearse DJ. Reperfusion-induced arrhythmias: mechanisms and prevention. J Mol Cell Cardiol. 1984;16:497–518. - PubMed
-
- Prinzmetal M, Kennamer R, Merliss R, Wada T, Naci B. Angina pectoris. I. A variant form of angina pectoris. Am J Med. 1959;27:375–388. - PubMed
-
- Unstable angina pectoris: national co-operative study group to compare surgical and medical therapy. Results in patients with S-T segment elevation during pain. Am J Cardiol. 1980;45:819–823. - PubMed
-
- Keller KB, Lemberg L. Prinzmetal's angina. Am J Crit Care. 2004;13:350–354. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

