Prognostic and predictive gene signature for adjuvant chemotherapy in resected non-small-cell lung cancer
- PMID: 20823422
- PMCID: PMC2988634
- DOI: 10.1200/JCO.2009.26.4325
Prognostic and predictive gene signature for adjuvant chemotherapy in resected non-small-cell lung cancer
Erratum in
- J Clin Oncol. 2010 Dec 1;28(34):5126
- J Clin Oncol. 2014 Feb 1;32(4):365
Abstract
Purpose: The JBR.10 trial demonstrated benefit from adjuvant cisplatin/vinorelbine (ACT) in early-stage non-small-cell lung cancer (NSCLC). We hypothesized that expression profiling may identify stage-independent subgroups who might benefit from ACT.
Patients and methods: Gene expression profiling was conducted on mRNA from 133 frozen JBR.10 tumor samples (62 observation [OBS], 71 ACT). The minimum gene set that was selected for the greatest separation of good and poor prognosis patient subgroups in OBS patients was identified. The prognostic value of this gene signature was tested in four independent published microarray data sets and by quantitative reverse-transcriptase polymerase chain reaction (RT-qPCR).
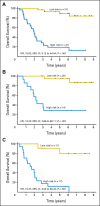
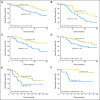
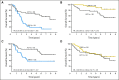
Results: A 15-gene signature separated OBS patients into high-risk and low-risk subgroups with significantly different survival (hazard ratio [HR], 15.02; 95% CI, 5.12 to 44.04; P < .001; stage I HR, 13.31; P < .001; stage II HR, 13.47; P < .001). The prognostic effect was verified in the same 62 OBS patients where gene expression was assessed by qPCR. Furthermore, it was validated consistently in four separate microarray data sets (total 356 stage IB to II patients without adjuvant treatment) and additional JBR.10 OBS patients by qPCR (n = 19). The signature was also predictive of improved survival after ACT in JBR.10 high-risk patients (HR, 0.33; 95% CI, 0.17 to 0.63; P = .0005), but not in low-risk patients (HR, 3.67; 95% CI, 1.22 to 11.06; P = .0133; interaction P < .001). Significant interaction between risk groups and ACT was verified by qPCR.
Conclusion: This 15-gene expression signature is an independent prognostic marker in early-stage, completely resected NSCLC, and to our knowledge, is the first signature that has demonstrated the potential to select patients with stage IB to II NSCLC most likely to benefit from adjuvant chemotherapy with cisplatin/vinorelbine.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Non-small-cell lung cancer mRNA expression signature predicting response to adjuvant chemotherapy.J Clin Oncol. 2010 Oct 10;28(29):4404-7. doi: 10.1200/JCO.2010.31.0144. Epub 2010 Sep 7. J Clin Oncol. 2010. PMID: 20823415 No abstract available.
References
-
- Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352:2589–2597. - PubMed
-
- Pisters KM, Evans WK, Azzoli CG, et al. Cancer Care Ontario and American Society of Clinical Oncology adjuvant chemotherapy and adjuvant radiation therapy for stages I-IIIA resectable non small-cell lung cancer guideline. J Clin Oncol. 2007;25:5506–5518. - PubMed
-
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–3559. - PubMed
-
- Nesbitt JC, Putnam JB, Jr, Walsh GL, et al. Survival in early-stage non-small cell lung cancer. Ann Thorac Surg. 1995;60:466–472. - PubMed
-
- Beer DG, Kardia SL, Huang CC, et al. Gene-expression profiles predict survival of patients with lung adenocarcinoma. Nat Med. 2002;8:816–824. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

