Reuse of nevirapine in exposed HIV-infected children after protease inhibitor-based viral suppression: a randomized controlled trial
- PMID: 20823434
- PMCID: PMC4540068
- DOI: 10.1001/jama.2010.1278
Reuse of nevirapine in exposed HIV-infected children after protease inhibitor-based viral suppression: a randomized controlled trial
Abstract
Context: Protease inhibitor (PI)-based therapy is recommended for infants infected with human immunodeficiency virus (HIV) who were exposed to nevirapine for prevention of mother-to-child HIV transmission. However, there are limitations of continuing PI-based therapy indefinitely and reuse of nevirapine has many advantages.
Objective: To test whether nevirapine-exposed infants who initially achieve viral suppression with PI-based therapy can maintain viral suppression when switched to nevirapine-based therapy.
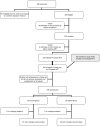
Design, setting, and patients: Randomized trial conducted between April 2005 and May 2009 at a hospital in Johannesburg, South Africa, among 195 children who achieved viral suppression less than 400 copies/mL for 3 or more months from a cohort of 323 nevirapine-exposed children who initiated PI-based therapy before 24 months of age.
Interventions: Control group children continued to receive ritonavir-boosted lopinavir, stavudine, and lamivudine (n = 99). Switch group children substituted nevirapine for ritonavir-boosted lopinavir (n = 96).
Main outcome measures: Children were followed up for 52 weeks after randomization. Plasma HIV-1 RNA of greater than 50 copies/mL was the primary end point. Confirmed viremia greater than 1000 copies/mL was used as a criterion to consider regimen changes for children in either group (safety end point).
Results: Plasma viremia greater than 50 copies/mL occurred less frequently in the switch group (Kaplan-Meier probability, 0.438; 95% CI, 0.334-0.537) than in the control group (0.576; 95% CI, 0.470-0.668) (P = .02). Confirmed viremia greater than 1000 copies/mL occurred more frequently in the switch group (0.201; 95% CI, 0.125-0.289) than in the control group (0.022; 95% CI, 0.004-0.069) (P < .001). CD4 cell response was better in the switch group (median CD4 percentage at 52 weeks, 34.7) vs the control group (CD4 percentage, 31.3) (P = .004). Older age (relative hazard [RH], 1.71; 95% CI, 1.08-2.72) was associated with viremia greater than 50 copies/mL in the control group. Inadequate adherence (RH, 4.14; 95% CI, 1.18-14.57) and drug resistance (RH, 4.04; 95% CI, 1.40-11.65) before treatment were associated with confirmed viremia greater than 1000 copies/mL in the switch group.
Conclusion: Among HIV-infected children previously exposed to nevirapine, switching to nevirapine-based therapy after achieving viral suppression with a ritonavir-boosted lopinavir regimen resulted in lower rates of viremia greater than 50 copies/mL than maintaining the primary ritonavir-boosted lopinavir regimen.
Trial registration: clinicaltrials.gov Identifier: NCT00117728.
Figures
Comment in
-
Antiretroviral treatment for HIV-infected infants exposed to nevirapine.JAMA. 2010 Dec 15;304(23):2589; author reply 2589-90. doi: 10.1001/jama.2010.1818. JAMA. 2010. PMID: 21156944 No abstract available.
References
-
- Arrive E, Newell ML, Ekouevi DK, et al. Prevalence of resistance to nevirapine in mothers and children after single-dose exposure to prevent vertical transmission of HIV-1: a meta-analysis. Int J Epidemiol. 2007;36:1009–1021. - PubMed
-
- Martinson NA, Morris L, Gray G, et al. Selection and persistence of viral resistance in HIV-infected children after exposure to single-dose nevirapine. J Acquir Immune Defic Syndr. 2007;44:148–153. - PubMed
-
- Lockman S, Shapiro RL, Smeaton LM, et al. Response to antiretroviral therapy after a single, peripartum dose of nevirapine. N Engl J Med. 2007;356:135–147. - PubMed
-
- Palumbo P, Violari A, Lindsey J, et al. Nevirapine (NVP) vs lopinavir-ritonavir (LPV/r)-based antiretroviral therapy (ART) in single dose nevirapine (sdNVP)-exposed HIV-infected infants: preliminary results from the IMPAACT P1060 trial. 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention. 2009; 19-22 July 2009; Cape Town, South Africa.
-
- Lockman S A5208/OCTANE Study Team. Lopinavir/ritonavir+Tenofovir/Emtricitabine is superior to Nevirapine+Tenofovir/Emtricitabine for women with prior exposure to single-dose nevirapine. 16th Conference on Retroviruses and Opportunistic Infections. 2009; Feb 8-11; Montreal, Canada.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous