Fabrication of three-dimensional porous cell-laden hydrogel for tissue engineering
- PMID: 20823504
- PMCID: PMC3282162
- DOI: 10.1088/1758-5082/2/3/035003
Fabrication of three-dimensional porous cell-laden hydrogel for tissue engineering
Abstract
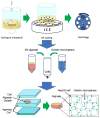
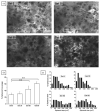
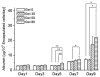
For tissue engineering applications, scaffolds should be porous to enable rapid nutrient and oxygen transfer while providing a three-dimensional (3D) microenvironment for the encapsulated cells. This dual characteristic can be achieved by fabrication of porous hydrogels that contain encapsulated cells. In this work, we developed a simple method that allows cell encapsulation and pore generation inside alginate hydrogels simultaneously. Gelatin beads of 150-300 microm diameter were used as a sacrificial porogen for generating pores within cell-laden hydrogels. Gelation of gelatin at low temperature (4 degrees C) was used to form beads without chemical crosslinking and their subsequent dissolution after cell encapsulation led to generation of pores within cell-laden hydrogels. The pore size and porosity of the scaffolds were controlled by the gelatin bead size and their volume ratio, respectively. Fabricated hydrogels were characterized for their internal microarchitecture, mechanical properties and permeability. Hydrogels exhibited a high degree of porosity with increasing gelatin bead content in contrast to nonporous alginate hydrogel. Furthermore, permeability increased by two to three orders while compressive modulus decreased with increasing porosity of the scaffolds. Application of these scaffolds for tissue engineering was tested by encapsulation of hepatocarcinoma cell line (HepG2). All the scaffolds showed similar cell viability; however, cell proliferation was enhanced under porous conditions. Furthermore, porous alginate hydrogels resulted in formation of larger spheroids and higher albumin secretion compared to nonporous conditions. These data suggest that porous alginate hydrogels may have provided a better environment for cell proliferation and albumin production. This may be due to the enhanced mass transfer of nutrients, oxygen and waste removal, which is potentially beneficial for tissue engineering and regenerative medicine applications.
Figures



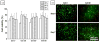

Similar articles
-
In situ generation of cell-laden porous MMP-sensitive PEGDA hydrogels by gelatin leaching.Macromol Biosci. 2014 May;14(5):731-9. doi: 10.1002/mabi.201300406. Epub 2014 Jan 20. Macromol Biosci. 2014. PMID: 24443002
-
Alginate dependent changes of physical properties in 3D bioprinted cell-laden porous scaffolds affect cell viability and cell morphology.Biomed Mater. 2019 Sep 25;14(6):065009. doi: 10.1088/1748-605X/ab3c74. Biomed Mater. 2019. PMID: 31426033
-
Three dimensional printed bioglass/gelatin/alginate composite scaffolds with promoted mechanical strength, biomineralization, cell responses and osteogenesis.J Mater Sci Mater Med. 2020 Aug 20;31(9):77. doi: 10.1007/s10856-020-06413-6. J Mater Sci Mater Med. 2020. PMID: 32816067
-
Macroporous Hydrogel Scaffolds for Three-Dimensional Cell Culture and Tissue Engineering.Tissue Eng Part B Rev. 2017 Oct;23(5):451-461. doi: 10.1089/ten.TEB.2016.0465. Epub 2017 Feb 3. Tissue Eng Part B Rev. 2017. PMID: 28067115 Review.
-
Design properties of hydrogel tissue-engineering scaffolds.Expert Rev Med Devices. 2011 Sep;8(5):607-26. doi: 10.1586/erd.11.27. Expert Rev Med Devices. 2011. PMID: 22026626 Free PMC article. Review.
Cited by
-
Hydrogel Scaffolds to Deliver Cell Therapies for Wound Healing.Front Bioeng Biotechnol. 2021 May 3;9:660145. doi: 10.3389/fbioe.2021.660145. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34012956 Free PMC article. Review.
-
Agar-tragacanth/silk fibroin hydrogel containing Zn-based MOF as a novel nanobiocomposite with biological activity.Sci Rep. 2024 May 7;14(1):10508. doi: 10.1038/s41598-024-61329-3. Sci Rep. 2024. PMID: 38714808 Free PMC article.
-
The one-step fabrication of porous hASC-laden GelMa constructs using a handheld printing system.NPJ Regen Med. 2023 Jun 10;8(1):30. doi: 10.1038/s41536-023-00307-1. NPJ Regen Med. 2023. PMID: 37301902 Free PMC article.
-
3D Bioprinting of Oxygenated Cell-Laden Gelatin Methacryloyl Constructs.Adv Healthc Mater. 2020 Aug;9(15):e1901794. doi: 10.1002/adhm.201901794. Epub 2020 Jun 16. Adv Healthc Mater. 2020. PMID: 32548961 Free PMC article.
-
Electrospray-Based Microencapsulation of Epigallocatechin 3-Gallate for Local Delivery into the Intervertebral Disc.Pharmaceutics. 2019 Sep 1;11(9):435. doi: 10.3390/pharmaceutics11090435. Pharmaceutics. 2019. PMID: 31480533 Free PMC article.
References
-
- Choi NW, Cabodi M, Held B, Gleghorn JP, Bonassar LJ, Stroock AD. Microfluidic scaffolds for tissue engineering. Nat Mater. 2007;6:908–15. - PubMed
-
- Khetani SR, Bhatia SN. Microscale culture of human liver cells for drug development. Nat Biotechnol. 2008;26:120–6. - PubMed
-
- Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater. 2005;4:518–24. - PubMed
-
- Huang X, Zhang Y, Donahue HJ, Lowe TL. Porous thermoresponsive-co-biodegradable hydrogels as tissue-engineering scaffolds for 3-dimensional in vitro culture of chondrocytes. Tissue Eng. 2007;13:2645–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
