Mutations in the c-Kit gene disrupt mitogen-activated protein kinase signaling during tumor development in adenoid cystic carcinoma of the salivary glands
- PMID: 20824047
- PMCID: PMC2933691
- DOI: 10.1593/neo.10356
Mutations in the c-Kit gene disrupt mitogen-activated protein kinase signaling during tumor development in adenoid cystic carcinoma of the salivary glands
Abstract
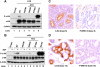
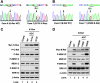
The Ras/mitogen-activated protein kinase (MAPK) pathway is considered to be a positive regulator of tumor initiation, progression, and maintenance. This study reports an opposite finding: we have found strong evidence that the MAPK pathway is inhibited in a subset of adenoid cystic carcinomas (ACCs) of the salivary glands. ACC tumors consistently overexpress the receptor tyrosine kinase (RTK) c-Kit, which has been considered a therapeutic target. We performed mutational analysis of the c-Kit gene (KIT in 17 cases of ACC and found that 2 cases of ACC had distinct missense mutations in KIT at both the genomic DNA and messenger RNA levels. These mutations caused G664R and R796G amino acid substitutions in the kinase domains. Surprisingly, the mutations were functionally inactive in cultured cells. We observed a significant reduction of MAPK (ERK1/2) activity in tumor cells, as assessed by immunohistochemistry. We performed further mutational analysis of the downstream effectors in the c-Kit pathway in the genes HRAS, KRAS, NRAS, BRAF, PIK3CA, and PTEN. This analysis revealed that two ACC tumors without KIT mutations had missense mutations in either KRAS or BRAF, causing S17N K-Ras and V590I B-Raf mutants, respectively. Our functional analysis showed that proteins with these mutations were also inactive in cultured cells. This is the first time that MAPK activity from the RTK signaling has been shown to be inhibited by gene mutations during tumor development. Because ACC seems to proliferate despite inactivation of the c-Kit signaling pathway, we suggest that selective inhibition of c-Kit is probably not a suitable treatment strategy for ACC.
Figures

References
-
- Spiro RH, Huvos AG, Strong EW. Adenoid cystic carcinoma of salivary origin. A clinicopathologic study of 242 cases. Am J Surg. 1974;128:512–520. - PubMed
-
- Carlson ER, Kim J, Mirowski GW, Stewart JCB, Zarbo RJ. Salivary gland diseases. In: Regezi JA, Sciubba JJ, Jordan RCK, editors. Oral Pathology: Clinical Pathology Correlations. St Louis, MO: Saunders; 2008. pp. 179–215.
-
- Chen AM, Garcia J, Lee NY, Bucci MK, Eisele DW. Patterns of nodal relapse after surgery and postoperative radiation therapy for carcinomas of the major and minor salivary glands: what is the role of elective neck irradiation? Int J Radiat Oncol Biol Phys. 2007;67:988–994. - PubMed
-
- Laurie SA, Licitra L. Systemic therapy in the palliative management of advanced salivary gland cancers. J Clin Oncol. 2006;24:2673–2678. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
