Mapracorat, a novel selective glucocorticoid receptor agonist, inhibits hyperosmolar-induced cytokine release and MAPK pathways in human corneal epithelial cells
- PMID: 20824100
- PMCID: PMC2932489
Mapracorat, a novel selective glucocorticoid receptor agonist, inhibits hyperosmolar-induced cytokine release and MAPK pathways in human corneal epithelial cells
Abstract
Purpose: Increasing evidence suggests that tear hyperosmolarity is a central mechanism causing ocular surface inflammation and damage in dry eye disease. Mapracorat (BOL-303242-X) is a novel glucocorticoid receptor agonist currently under clinical evaluation for use in the treatment of dry eye disease. This study assessed the anti-inflammatory effects of mapracorat in an in vitro osmotic stress model which mimics some of the pathophysiological changes seen in dry eye.
Methods: Human corneal epithelial cells were cultured in normal osmolar media (317 mOsM) or 440 mOsM hyperosmolar media for 24 h. Luminex technology was used to determine the effect of mapracorat on hyperosmolar-induced cytokine release. Effects of mapracorat on mitogen-activated protein kinase (MAPK) phosphorylation were determined by cell based ELISA. Effects of mapracorat on nuclear factor kappa B (NFkappaB) and activator protein-1 (AP-1) transcriptional activity were assessed by reporter gene assay. Dexamethasone was used as a control.
Results: Hyperosmolar conditions induced release of the pro-inflammatory cytokines interleukin-6 (IL-6), interleukin-8 (IL-8), and monocyte chemotactic protein-1 (MCP-1) from cultured human corneal epithelial cells, and altered the phosphorylation state of p38 and c-Jun N-terminal kinase (JNK) and transcriptional activity of NFkappaB and AP-1. Incubation of cells with mapracorat inhibited hyperosmolar-induced cytokine release with comparable activity and potency as dexamethasone. This inhibition was reversed by the glucocorticoid receptor antagonist mifepristone (RU-486). Increased phosphorylation of p38 and JNK caused by hyperosmolarity was inhibited by mapracorat. Mapracorat also significantly decreased the hyperosmolar-induced increase in NFkappaB and AP-1 transcriptional activity.
Conclusions: Mapracorat acts as a potent anti-inflammatory agent in corneal epithelial cells challenged with osmotic stress, with comparable activity to the traditional steroid dexamethasone. These in vitro data suggest that mapracorat may be efficacious in the treatment of dry eye disease.
Figures
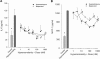




Similar articles
-
Anti-inflammatory and anti-oxidative effects of the green tea polyphenol epigallocatechin gallate in human corneal epithelial cells.Mol Vis. 2011 Feb 18;17:533-42. Mol Vis. 2011. PMID: 21364905 Free PMC article.
-
Eosinophil as a cellular target of the ocular anti-allergic action of mapracorat, a novel selective glucocorticoid receptor agonist.Mol Vis. 2011;17:3208-23. Epub 2011 Dec 14. Mol Vis. 2011. PMID: 22194647 Free PMC article.
-
BOL-303242-X, a novel selective glucocorticoid receptor agonist, with full anti-inflammatory properties in human ocular cells.Mol Vis. 2009 Dec 8;15:2606-16. Mol Vis. 2009. PMID: 20011631 Free PMC article.
-
Mapracorat, a selective glucocorticoid receptor agonist, upregulates RelB, an anti-inflammatory nuclear factor-kappaB protein, in human ocular cells.Exp Eye Res. 2014 Oct;127:290-8. doi: 10.1016/j.exer.2014.07.013. Exp Eye Res. 2014. PMID: 25245083
-
Mapracorat, a novel non-steroidal selective glucocorticoid receptor agonist for the treatment of allergic conjunctivitis.Inflamm Allergy Drug Targets. 2014;13(5):289-98. doi: 10.2174/1871528113666141106101356. Inflamm Allergy Drug Targets. 2014. PMID: 25373600 Review.
Cited by
-
Anti-allergic effects of mapracorat, a novel selective glucocorticoid receptor agonist, in human conjunctival fibroblasts and epithelial cells.Mol Vis. 2013 Jul 19;19:1515-25. Print 2013. Mol Vis. 2013. PMID: 23878502 Free PMC article.
-
In Vitro Inhibition of NFAT5-Mediated Induction of CCL2 in Hyperosmotic Conditions by Cyclosporine and Dexamethasone on Human HeLa-Modified Conjunctiva-Derived Cells.PLoS One. 2016 Aug 3;11(8):e0159983. doi: 10.1371/journal.pone.0159983. eCollection 2016. PLoS One. 2016. PMID: 27486749 Free PMC article.
-
Therapeutic targeting of eosinophil adhesion and accumulation in allergic conjunctivitis.Front Pharmacol. 2012 Dec 26;3:203. doi: 10.3389/fphar.2012.00203. eCollection 2012. Front Pharmacol. 2012. PMID: 23271999 Free PMC article.
-
Anti-inflammatory and anti-oxidative effects of the green tea polyphenol epigallocatechin gallate in human corneal epithelial cells.Mol Vis. 2011 Feb 18;17:533-42. Mol Vis. 2011. PMID: 21364905 Free PMC article.
-
Eosinophil as a cellular target of the ocular anti-allergic action of mapracorat, a novel selective glucocorticoid receptor agonist.Mol Vis. 2011;17:3208-23. Epub 2011 Dec 14. Mol Vis. 2011. PMID: 22194647 Free PMC article.
References
-
- DEWS report The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye Workshop (2007). Ocul Surf. 2007;5:75–92. - PubMed
-
- Bron AJ, Yokoi N, Gafney E, Tiffany JM. Predicted phenotypes of dry eye: Proposed consequences of its natural history. Ocul Surf. 2009;7:78–92. - PubMed
-
- Gilbard JP. Human tear film electrolyte concentrations in health and dry-eye disease. Int Ophthalmol Clin. 1994;34:27–36. - PubMed
-
- Liu H, Begley C, Chen M, Bradley A, Bonanno J, McNamara NA, Nelson JD, Simpson T. A link between tear instability and hyperosmolarity in dry eye. Invest Ophthalmol Vis Sci. 2009;50:3671–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
