Inhibition of GSK-3 ameliorates Abeta pathology in an adult-onset Drosophila model of Alzheimer's disease
- PMID: 20824130
- PMCID: PMC2932684
- DOI: 10.1371/journal.pgen.1001087
Inhibition of GSK-3 ameliorates Abeta pathology in an adult-onset Drosophila model of Alzheimer's disease
Erratum in
- PLoS Genet. 2012 Jan;8(1). doi: 10.1371/annotation/baa8a2a9-130b-4959-b6fb-6f786fd02826
Abstract
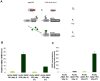
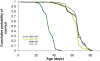
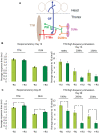
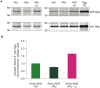
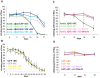
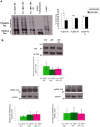
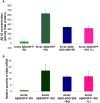
Abeta peptide accumulation is thought to be the primary event in the pathogenesis of Alzheimer's disease (AD), with downstream neurotoxic effects including the hyperphosphorylation of tau protein. Glycogen synthase kinase-3 (GSK-3) is increasingly implicated as playing a pivotal role in this amyloid cascade. We have developed an adult-onset Drosophila model of AD, using an inducible gene expression system to express Arctic mutant Abeta42 specifically in adult neurons, to avoid developmental effects. Abeta42 accumulated with age in these flies and they displayed increased mortality together with progressive neuronal dysfunction, but in the apparent absence of neuronal loss. This fly model can thus be used to examine the role of events during adulthood and early AD aetiology. Expression of Abeta42 in adult neurons increased GSK-3 activity, and inhibition of GSK-3 (either genetically or pharmacologically by lithium treatment) rescued Abeta42 toxicity. Abeta42 pathogenesis was also reduced by removal of endogenous fly tau; but, within the limits of detection of available methods, tau phosphorylation did not appear to be altered in flies expressing Abeta42. The GSK-3-mediated effects on Abeta42 toxicity appear to be at least in part mediated by tau-independent mechanisms, because the protective effect of lithium alone was greater than that of the removal of tau alone. Finally, Abeta42 levels were reduced upon GSK-3 inhibition, pointing to a direct role of GSK-3 in the regulation of Abeta42 peptide level, in the absence of APP processing. Our study points to the need both to identify the mechanisms by which GSK-3 modulates Abeta42 levels in the fly and to determine if similar mechanisms are present in mammals, and it supports the potential therapeutic use of GSK-3 inhibitors in AD.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Selkoe DJ, Schenk D. Alzheimer's disease: molecular understanding predicts amyloid-based therapeutics. Annu Rev Pharmacol Toxicol. 2003;43:545–584. - PubMed
-
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. - PubMed
-
- Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–1491. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

