The effects of aging on the molecular and cellular composition of the prostate microenvironment
- PMID: 20824135
- PMCID: PMC2931699
- DOI: 10.1371/journal.pone.0012501
The effects of aging on the molecular and cellular composition of the prostate microenvironment
Abstract
Background: Advancing age is associated with substantial increases in the incidence rates of common diseases affecting the prostate gland including benign prostatic hyperplasia (BPH) and prostate carcinoma. The prostate is comprised of a functional secretory epithelium, a basal epithelium, and a supporting stroma comprised of structural elements, and a spectrum of cell types that includes smooth muscle cells, fibroblasts, and inflammatory cells. As reciprocal interactions between epithelium and stromal constituents are essential for normal organogenesis and serve to maintain normal functions, discordance within the stroma could permit or promote disease processes. In this study we sought to identify aging-associated alterations in the mouse prostate microenvironment that could influence pathology.
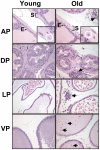
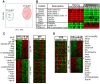
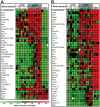
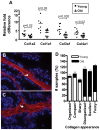
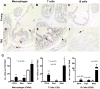
Methodology/principal findings: We quantitated transcript levels in microdissected glandular-adjacent stroma from young (age 4 months) and old (age 20-24 months) C57BL/6 mice, and identified a significant change in the expression of 1259 genes (p<0.05). These included increases in transcripts encoding proteins associated with inflammation (e.g., Ccl8, Ccl12), genotoxic/oxidative stress (e.g., Apod, Serpinb5) and other paracrine-acting effects (e.g., Cyr61). The expression of several collagen genes (e.g., Col1a1 and Col3a1) exhibited age-associated declines. By histology, immunofluorescence, and electron microscopy we determined that the collagen matrix is abundant and disorganized, smooth muscle cell orientation is disordered, and inflammatory infiltrates are significantly increased, and are comprised of macrophages, T cells and, to a lesser extent, B cells.
Conclusion/significance: These findings demonstrate that during normal aging the prostate stroma exhibits phenotypic and molecular characteristics plausibly contributing to the striking age associated pathologies affecting the prostate.
Conflict of interest statement
Figures


Similar articles
-
Hyaluronan in aged collagen matrix increases prostate epithelial cell proliferation.In Vitro Cell Dev Biol Anim. 2015 Jan;51(1):50-8. doi: 10.1007/s11626-014-9800-z. Epub 2014 Aug 15. In Vitro Cell Dev Biol Anim. 2015. PMID: 25124870 Free PMC article.
-
Role of stroma in carcinogenesis of the prostate.Differentiation. 2002 Dec;70(9-10):473-85. doi: 10.1046/j.1432-0436.2002.700902.x. Differentiation. 2002. PMID: 12492490 Review.
-
Age-related changes in guinea pig prostatic stroma.Lab Invest. 1994 May;70(5):753-63. Lab Invest. 1994. PMID: 8196369
-
Proliferation of Prostate Stromal Cell Induced by Benign Prostatic Hyperplasia Epithelial Cell Stimulated With Trichomonas vaginalis via Crosstalk With Mast Cell.Prostate. 2016 Nov;76(15):1431-44. doi: 10.1002/pros.23227. Epub 2016 Jun 21. Prostate. 2016. PMID: 27325623
-
Role of the stromal microenvironment in carcinogenesis of the prostate.Int J Cancer. 2003 Oct 20;107(1):1-10. doi: 10.1002/ijc.11335. Int J Cancer. 2003. PMID: 12925950 Review.
Cited by
-
E-cadherin deficiency promotes prostate macrophage inflammation and bladder overactivity in aged male mice.Aging (Albany NY). 2022 Mar 31;14(7):2945-2965. doi: 10.18632/aging.203994. Epub 2022 Mar 31. Aging (Albany NY). 2022. PMID: 35361739 Free PMC article.
-
Antiangiogenic therapy effects on age-associated matrix metalloproteinase-9 (MMP-9) and insulin-like growth factor receptor-1 (IGFR-1) responses: a comparative study of prostate disorders in aged and TRAMP mice.Histochem Cell Biol. 2014 Sep;142(3):269-84. doi: 10.1007/s00418-014-1193-2. Epub 2014 Feb 22. Histochem Cell Biol. 2014. PMID: 24562790
-
Psychosocial Stress and Age Influence Depression and Anxiety-Related Behavior, Drive Tumor Inflammatory Cytokines and Accelerate Prostate Cancer Growth in Mice.Front Oncol. 2021 Sep 16;11:703848. doi: 10.3389/fonc.2021.703848. eCollection 2021. Front Oncol. 2021. PMID: 34604038 Free PMC article.
-
Potential protective mechanisms of aryl hydrocarbon receptor (AHR) signaling in benign prostatic hyperplasia.Differentiation. 2011 Nov-Dec;82(4-5):211-9. doi: 10.1016/j.diff.2011.05.011. Differentiation. 2011. PMID: 21684673 Free PMC article. Review.
-
Distinct cell-types in the prostate share an aging signature suggestive of metabolic reprogramming.Am J Clin Exp Urol. 2020 Aug 15;8(4):140-151. eCollection 2020. Am J Clin Exp Urol. 2020. PMID: 32929410 Free PMC article.
References
-
- Davidovic M. Genetic stability: the key to longevity? Med Hypotheses. 1999;53:329–332. - PubMed
-
- Baylin SB, Herman JG, Graff JR, Vertino PM, Issa JP. Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv Cancer Res. 1998;72:141–196. - PubMed
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

