Gene knock-outs of inositol 1,4,5-trisphosphate receptors types 1 and 2 result in perturbation of cardiogenesis
- PMID: 20824138
- PMCID: PMC2931702
- DOI: 10.1371/journal.pone.0012500
Gene knock-outs of inositol 1,4,5-trisphosphate receptors types 1 and 2 result in perturbation of cardiogenesis
Abstract
Background: Inositol 1,4,5-trisphosphate receptors (IP3R1, 2, and 3) are intracellular Ca2+ release channels that regulate various vital processes. Although the ryanodine receptor type 2, another type of intracellular Ca2+ release channel, has been shown to play a role in embryonic cardiomyocytes, the functions of the IP3Rs in cardiogenesis remain unclear.
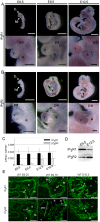
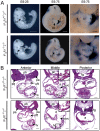
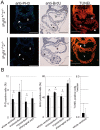
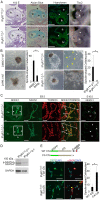
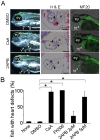
Methodology/principal findings: We found that IP3R1(-/-)-IP3R2(-/-) double-mutant mice died in utero with developmental defects of the ventricular myocardium and atrioventricular (AV) canal of the heart by embryonic day (E) 11.5, even though no cardiac defect was detectable in IP3R1(-/-) or IP3R2(-/-) single-mutant mice at this developmental stage. The double-mutant phenotype resembled that of mice deficient for calcineurin/NFATc signaling, and NFATc was inactive in embryonic hearts from the double knockout-mutant mice. The double mutation of IP3R1/R2 and pharmacologic inhibition of IP3Rs mimicked the phenotype of the AV valve defect that result from the inhibition of calcineurin, and it could be rescued by constitutively active calcineurin.
Conclusions/significance: Our results suggest an essential role for IP3Rs in cardiogenesis in part through the regulation of calcineurin-NFAT signaling.
Conflict of interest statement
Figures
References
-
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. - PubMed
-
- Furuichi T, Yoshikawa S, Miyawaki A, Wada K, Maeda N, et al. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature. 1989;342:32–38. - PubMed
-
- Iwai M, Tateishi Y, Hattori M, Mizutani A, Nakamura T, et al. Molecular cloning of mouse type 2 and type 3 inositol 1,4,5-trisphosphate receptors and identification of a novel type 2 receptor splice variant. J Biol Chem. 2005;280:10305–10317. - PubMed
-
- Matsumoto M, Nakagawa T, Inoue T, Nagata E, Tanaka K, et al. Ataxia and epileptic seizures in mice lacking type 1 inositol 1,4,5-trisphosphate receptor. Nature. 1996;379:168–171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

