The perilipin homologue, lipid storage droplet 2, regulates sleep homeostasis and prevents learning impairments following sleep loss
- PMID: 20824166
- PMCID: PMC2930866
- DOI: 10.1371/journal.pbio.1000466
The perilipin homologue, lipid storage droplet 2, regulates sleep homeostasis and prevents learning impairments following sleep loss
Abstract
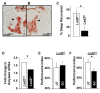
Extended periods of waking result in physiological impairments in humans, rats, and flies. Sleep homeostasis, the increase in sleep observed following sleep loss, is believed to counter the negative effects of prolonged waking by restoring vital biological processes that are degraded during sleep deprivation. Sleep homeostasis, as with other behaviors, is influenced by both genes and environment. We report here that during periods of starvation, flies remain spontaneously awake but, in contrast to sleep deprivation, do not accrue any of the negative consequences of prolonged waking. Specifically, the homeostatic response and learning impairments that are a characteristic of sleep loss are not observed following prolonged waking induced by starvation. Recently, two genes, brummer (bmm) and Lipid storage droplet 2 (Lsd2), have been shown to modulate the response to starvation. bmm mutants have excess fat and are resistant to starvation, whereas Lsd2 mutants are lean and sensitive to starvation. Thus, we hypothesized that bmm and Lsd2 may play a role in sleep regulation. Indeed, bmm mutant flies display a large homeostatic response following sleep deprivation. In contrast, Lsd2 mutant flies, which phenocopy aspects of starvation as measured by low triglyceride stores, do not exhibit a homeostatic response following sleep loss. Importantly, Lsd2 mutant flies are not learning impaired after sleep deprivation. These results provide the first genetic evidence, to our knowledge, that lipid metabolism plays an important role in regulating the homeostatic response and can protect against neuronal impairments induced by prolonged waking.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Van Cauter E, Holmback U, Knutson K, Leproult R, Miller A, et al. Impact of sleep and sleep loss on neuroendocrine and metabolic function. Horm Res. 2007;67(Suppl 1):2–9. - PubMed
-
- Graves L. A, Hellman K, Veasey S, Blendy J. A, Pack A. I, et al. Genetic evidence for a role of CREB in sustained cortical arousal. J Neurophysiol. 2003;90:1152–1159. - PubMed
-
- Rechtschaffen A, Gilliland M. A, Bergmann B. M, Winter J. B. Physiological correlates of prolonged sleep deprivation in rats. Science. 1983;221:182–184. - PubMed
-
- Rogers N. L, Dorrian J, Dinges D. F. Sleep, waking and neurobehavioural performance. Front Biosci. 2003;8:s1056–s1067. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

