Efficacy and tolerability of high-dose dronabinol maintenance in HIV-positive marijuana smokers: a controlled laboratory study
- PMID: 20824270
- PMCID: PMC3325767
- DOI: 10.1007/s00213-010-1995-4
Efficacy and tolerability of high-dose dronabinol maintenance in HIV-positive marijuana smokers: a controlled laboratory study
Abstract
Rationale: Dronabinol (Δ(9)tetrahydrocannabinol) is approved for HIV-related anorexia, yet, little is known about its effects in HIV-positive marijuana smokers. HIV-negative marijuana smokers require higher than recommended dronabinol doses to experience expected effects.
Objectives: Employing a within-subjects, double-blind, placebo-controlled design, we assessed the effects of repeated high-dose dronabinol in HIV-positive marijuana smokers taking antiretroviral medication.
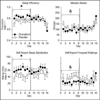
Methods: Participants (N = 7), who smoked marijuana 4.2 ± 2.3 days/week, resided in a residential laboratory for two 16-day stays, receiving dronabinol (10 mg QID) in one stay and placebo in the other. Efficacy was assessed with objectively verified food intake and body weight. Tolerability was measured with sleep, subjective, and cognitive assessments. For analyses, each inpatient stay was divided into two phases, days 1-8 and 9-16; we compared dronabinol's effects with placebo in each 8-day phase to investigate tolerance.
Results: Despite sustained increases in self-reported food cravings, dronabinol only increased caloric intake in the initial 8 days of dosing. Similarly, sleep quality was improved only in the first 8 days of dosing. Dronabinol's mood-enhancing effects were sustained across the 16-day inpatient stay. Dronabinol was well tolerated, causing few negative subjective or cognitive effects.
Conclusions: In HIV-positive marijuana smokers, high dronabinol doses safely and effectively increased caloric intake. However, repeated high-dose dronabinol appeared to result in selective tolerance to these effects. These findings indicate that HIV-positive individuals who smoke marijuana may require higher dronabinol doses than are recommended by the FDA. Future research to establish optimal dosing regimens, and reduce the development of tolerance, is required.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures



References
-
- Abrams DI. Medical marijuana: Trials and tribulations. J Psychoactive Drugs. 1998;30:163–169. - PubMed
-
- Abrams DI. Potential interventions for HIV/AIDS wasting: An overview. JAIDS. 2000;25:S74–S80. - PubMed
-
- Abrams DI, Hilton JF, Leiser RJ, Shade SB, Elbeik TA, Aweeka FT, Benowitz NL, Bredt BM, Kosel B, Aberg JA, Deeks SG, Mitchell TF, Mulligan K, Bacchetti P, McCune JM, Schambelan M. Short-term effects of cannabinoids in patients with HIV-1 infection. Ann Intern Med. 2003;139:258–266. - PubMed
-
- American Psychiatric Association. DSM-IV: Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association, American Psychiatric Association; 1994.
-
- Ashton CH. Pharmacology and effects of cannabis: A brief review. Br J Psychiatry. 2001;178:101–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

