First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas
- PMID: 20824724
- PMCID: PMC4665634
- DOI: 10.1002/cncr.25425
First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas
Abstract
Background: Temozolomide is an active agent in metastatic pancreatic endocrine carcinomas. In vitro data indicate that the combination of capecitabine and temozolomide is synergistic for induction of apoptosis in neuroendocrine tumor cell lines. The authors retrospectively evaluated the efficacy of capecitabine and temozolomide in 30 patients with metastatic pancreatic endocrine carcinomas to assess response rate, progression free survival (PFS), and overall survival (OS).
Methods: Patients with metastatic, well, or moderately differentiated pancreatic endocrine carcinomas who had not received prior systemic chemotherapy were treated with capecitabine (750 mg/m² twice daily, days 1-14) and temozolomide (200 mg/m² once daily, days 10-14) every 28 days.
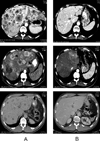
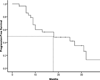
Results: Among 30 patients treated, 21 (70%) patients achieved an objective radiographic response. Median progression-free survival was 18 months. The rate of survival at two years was 92%. Only 4 patients (12%) experienced grade 3 or 4 adverse events.
Conclusions: The combination of capecitabine and temozolomide is associated with an exceptionally high and durable response rate in metastatic endocrine carcinomas of the pancreas. Clinical endpoints, including response rate, survival, and toxicity, are superior to those observed with streptozocin-based regimens.
Copyright © 2010 American Cancer Society.
Conflict of interest statement
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
Figures


References
-
- Oberg K, Eriksson B. Endocrine tumours of the pancreas. Best Pract Res Clin Gastroenterol. 2005;19:753–781. - PubMed
-
- Strosberg J, Gardner N, Kvols L. Survival and prognostic factor analysis in patients with metastatic pancreatic endocrine carcinomas. Pancreas. 2009;38:255–258. - PubMed
-
- Strosberg J, Nasir A, Coppola D, Wick M, Kvols L. Correlation between grade and prognosis in metastatic gastroenteropancreatic neuroendocrine tumors. Hum Pathol. 2009;40:1262–1268. - PubMed
-
- Moertel CG, Kvols LK, O’Connell MJ, Rubin J. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer. 1991;68:227–232. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

