L-asparaginase treatment in acute lymphoblastic leukemia: a focus on Erwinia asparaginase
- PMID: 20824725
- PMCID: PMC3000881
- DOI: 10.1002/cncr.25489
L-asparaginase treatment in acute lymphoblastic leukemia: a focus on Erwinia asparaginase
Abstract
Asparaginases are a cornerstone of treatment protocols for acute lymphoblastic leukemia (ALL) and are used for remission induction and intensification treatment in all pediatric regimens and in the majority of adult treatment protocols. Extensive clinical data have shown that intensive asparaginase treatment improves clinical outcomes in childhood ALL. Three asparaginase preparations are available: the native asparaginase derived from Escherichia coli (E. coli asparaginase), a pegylated form of this enzyme (PEG-asparaginase), and a product isolated from Erwinia chrysanthemi, ie, Erwinia asparaginase. Clinical hypersensitivity reactions and silent inactivation due to antibodies against E. coli asparaginase, lead to inactivation of E. coli asparaginase in up to 60% of cases. Current treatment protocols include E. coli asparaginase or PEG-asparaginase for first-line treatment of ALL. Typically, patients exhibiting sensitivity to one formulation of asparaginase are switched to another to ensure they receive the most efficacious treatment regimen possible. Erwinia asparaginase is used as a second- or third-line treatment in European and US protocols. Despite the universal inclusion of asparaginase in such treatment protocols, debate on the optimal formulation and dosage of these agents continues. This article provides an overview of available evidence for optimal use of Erwinia asparaginase in the treatment of ALL.
Copyright © 2010 American Cancer Society.
Conflict of interest statement
Pieters R is involved in scientific collaborations with different companies producing and developing asparaginases.
Hunger S is the Ergen Family Chair in Pediatric Cancer.
Boos J: Served personally as consultant and participated in advisory boards for different asparaginase-selling companies, including EUSA Pharma and former license holders. In addition, J Boos is also involved in scientific collaborations with different companies producing and developing asparaginase
Rizzari C. is involved in scientific researches supported by different companies producing and/or marketing asparaginase products.
Silverman L served on advisory board for EUSA Pharma and as a consultant for Enzon Pharmaceuticals.
Baruchel A had received honorarium for lecture from OPI
Gökbuget N is involved in scientific collaborations with different companies producing and developing asparaginases
Schrappe M is involved in scientific collaborations with different companies producing and developing asparaginases.
Pui CH had received honorarium for lecture from EUSA Pharma.
Figures
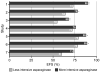
Silverman et al. (2)
Amylon et al. (38)
Amylon et al. (38)
Rizzari et al. (42)
Pession et al. (41)
Moghrabi et al. (40)
Duval et al. (39)
References
-
- Silverman LB, Declerck L, Gelber RD, Dalton VK, Asselin BL, Barr RD, et al. Results of Dana-Farber Cancer Institute Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1981-1995) Leukemia. 2000;14:2247–2256. - PubMed
-
- Silverman LB, Gelber RD, Dalton VK, Asselin BL, Barr RD, Clavell LA, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood. 2001;97:1211–1218. - PubMed
-
- Pui CH, Sandlund JT, Pei D, Campana D, Rivera GK, Ribeiro RC, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Total Therapy Study XIIIB at St Jude Children's Research Hospital. Blood. 2004;104:2690–2696. - PubMed
-
- Möricke A, Reiter A, Zimmermann M, Gadner H, Stanulla M, Dordelmann M, et al. Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood. 2008;111:4477–4489. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

