Formoterol versus short-acting beta-agonists as relief medication for adults and children with asthma
- PMID: 20824877
- PMCID: PMC4034434
- DOI: 10.1002/14651858.CD008418.pub2
Formoterol versus short-acting beta-agonists as relief medication for adults and children with asthma
Abstract
Background: Formoterol is a long-acting beta(2)-agonist but because it has a fast onset of action it can also be used as a relief medication.
Objectives: To asses the efficacy and safety of formoterol as reliever therapy in comparison to short-acting beta(2)-agonists in adults and children with asthma.
Search strategy: We searched the Cochrane Airways Group Specialised Register and websites of clinical trial registers (for unpublished trial data), and we checked the Food and Drug Administration (FDA) submissions in relation to formoterol. The date of the most recent search was February 2010.
Selection criteria: Randomised, parallel-arm trials of at least 12 weeks duration in patients of any age and severity of asthma. Studies randomised patients to any dose of as-needed formoterol versus short-acting beta(2)-agonist. Concomitant use of inhaled corticosteroids or other maintenance medication was allowed, as long as this was not part of the randomised treatment regimen.
Data collection and analysis: Two authors independently selected trials for inclusion in the review. Outcome data were extracted by one author and checked by the second author. We sought unpublished data on primary outcomes.
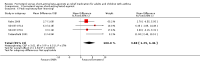
Main results: This review includes eight studies conducted in 22,604 participants (mostly adults). Six studies compared formoterol as-needed to terbutaline whilst two studies compared formoterol with salbutamol as-needed. Background maintenance therapy varied across the trials. Asthma exacerbations and serious adverse events showed a direction of treatment effect favouring formoterol, of which one outcome reached statistical significance (exacerbations requiring a course of oral corticosteroids). In patients on short-acting beta(2)-agonists, 117 people out of 1000 had exacerbations requiring oral corticosteroids over 30 weeks, compared to 101 (95% CI 93 to 108) out of 1000 for patients on formoterol as-needed. In patients on maintenance inhaled corticosteroids there were also significantly fewer exacerbations requiring a course of oral corticosteroids on formoterol as-needed (Peto OR 0.75; 95% CI 0.62 to 0.91). There was one death per 1000 people on formoterol or on short-acting beta(2)-agonists.
Authors' conclusions: In adults, formoterol was similar to short-acting beta(2)-agonists when used as a reliever, and showed a reduction in the number of exacerbations requiring a course of oral corticosteroids. Clinicians should weigh the relatively modest benefits of formoterol as-needed against the benefits of single inhaler therapy and the potential danger of long-term use of long-acting beta(2)-agonists in some patients. We did not find evidence to recommend changes to guidelines that suggest that long-acting beta(2)-agonists should be given only to patients already taking inhaled corticosteroids.There was insufficient information reported from children in the included trials to come to any conclusion on the safety or efficacy of formoterol as relief medication for children with asthma.
Conflict of interest statement
None known.
Figures





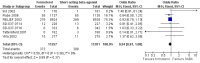

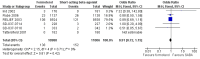








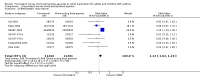



Update of
References
References to studies included in this review
Ind 2002 {published data only}
-
- Ind P, Borszormenyi Nagy G, Pietinalho A, Shiner R, Villasante C, Brander R, et al. Formoterol 4.5 microgram used as needed via turbuhaler was as safe and well tolerated as terbutaline 0.5 mg. European Respiratory Society, Oct 9‐13; Madrid, Spain. 1999:1086.
-
- Ind PW, Villasante C, Shiner RJ, Pietinalho A, Boszormenyi NG, Soliman S, et al. Safety of formoterol by Turbuhaler as reliever medication compared with terbutaline in moderate asthma. European Respiratory Journal 2002;20(4):859‐66. - PubMed
Jain 2004 {published data only}
-
- Jain A, Raghuram J. Randomized controlled study of the safety and efficacy of PRN formoterol compared to PRN albuterol for the management of asthma. American Thoracic Society 100th International Conference, May 21‐26, Orlando. 2004:B36 Poster G17.
Rabe 2006 {published data only}
-
- Rabe KF, Atienza T, Magyar P, Larsson P, Jorup C, Lalloo UG. Effect of budesonide in combination with formoterol for reliever therapy in asthma exacerbations: a randomised controlled, double‐blind study. Lancet 2006;368(9537):744‐53. - PubMed
-
- SD‐039‐0734. Efficay of Symbicort® Turbuhaler® 160/4.5 μg as needed versus Oxis® 4.5 μg as needed and Bricanyl® 0/4 mg as needed in adults and adolescents with asthma receiving Symbicort® Turbuhaler® 160/4.5 μg twice daily as maintenance treatment. A 12‐month, randomised, double‐blind, parallel group, active‐controlled, phase IIIB, multi‐centre study. AstraZeneca Study Report.
RELIEF 2003 {published data only}
-
- SD‐037‐0699. AstraZeneca Study Report.
-
- Lindgren B, Sears MR, Campbell M, Villasante C, Huang S, Lindh A, et al. Cost‐effectiveness of formoterol and salbutamol as asthma reliever medication in Sweden and in Spain. International Journal of Clinical Practice 2005;59(1):62‐8. - PubMed
-
- Lindgren B, Sears MR, Campbell M, Villasante C, Huang S, Lindh A, et al. Total costs according to reliever use of formoterol turbuhaler in asthma: results from the RELIEF worldwide randomised effectiveness study, stratified by maintenance medication levels. European Respiratory Journal 2002;20(Suppl 38):43s.
-
- Lindgren B, Sears MR, Compbell M, Villasante C, Huang S, Lindh A, et al. Cost‐effectiveness of formoterol turbuhaler versus salbutamol as reliever therapy in asthma: results from the RELIEF worldwide randomised effectiveness study. European Respiratory Journal. 2002:P389, 43s.
-
- Pauwels RA, Campbell M, Villasante C, Huang S, Lindh A, Petermann W. Formoterol turbuhaler compared with salbutamol as reliever medication in asthma: outcomes from the RELIEF study in patients across different severities and age groups. European Respiratory Journal. 2002; Vol. 20(Suppl 38):P395, 45s.
SD‐037‐0714 {published data only}
-
- A 12‐month comparison of Oxis® (formoterol) Turbuhaler® and Bricanyl® (terbutaline) Turbuhaler® both used as needed in patients with asthma on anti‐inflammatory treatment. AstraZeneca Study Report Vol. SD‐037‐0714.
-
- Chuchalin A, Kasl M, Bengtsson T, Nihlen U, Rosenborg J. Formoterol used as needed in patients with intermittent or mild persistent asthma. Respiratory Medicine 2005;99(4):461‐70. - PubMed
SD‐037‐0716 {published data only}
-
- A 12‐month comparison of Oxis® (formoterol) Turbuhaler® and Bricanyl® (terbutaline) Turbuhaler® both used as needed in patients with asthma not using anti‐inflammatory treatment. AstraZeneca Study Report Vol. SD‐037‐0716.
-
- Chuchalin A, Kasl M, Bengtsson T, Nihlen U, Rosenborg J. Formoterol used as needed in patients with intermittent or mild persistent asthma. Respiratory Medicine 2005;99(4):461‐70. - PubMed
-
- Chuchalin A, Makarova I, Bergqvist P. As‐needed formoterol has an improved safety profile compared with as‐needed terbutaline in mild intermittent asthma. European Respiratory Journal. 2003; Vol. 22(Suppl 45):P1571.
Tattersfield 2001 {published data only}
-
- Berggren F, Ekstrom T. A cost‐effectiveness study comparing the as‐needed use of formoterol Oxis and terbutaline Bricanyl in patients with moderate to severe asthma. Respiratory Medicine 2001;95(9):753‐8. - PubMed
-
- Berggren F, Ekstrom T. Formoterol was more cost effective in a modelling study than terbutaline in as needed treatment of patients with moderate asthma. European Respiratory Society. 1999:P2293.
-
- Berggren F, Svenson K, Rolnick M S, Alexander M, Mintz S. Twice‐daily formoterol Oxis® turbuhaler is more cost‐effective than twice‐daily salmeterol or as‐needed salbutamol. American Thoracic Society Meeting. 2001.
-
- Stahl E, Postma DS, Svensson K, Tattersfield AE, Eivindson A, Schreurs A, et al. Formoterol used as needed improves health‐related quality of life in asthmatic patients uncontrolled with inhaled corticosteroids. Respiratory Medicine 2003;96(9):1061‐6. - PubMed
-
- Tattersfield AE, Lofdahl CG, Postma DS, Eivindson A, Schreurs AG, Rasidakis A, et al. Comparison of formoterol and terbutaline for as‐needed treatment of asthma: a randomised trial. Lancet 2001;357(9252):257‐61. - PubMed
Villa 2002 {published data only}
-
- SD‐037‐0695. AstraZeneca Study report 2001.
-
- Villa J, Kuna P, Egner J, Brader R. Safety of formoterol reliever therapy compared with terbutaline in asthmatic children taking anti‐inflammatory therapy. European Respiratory Society Annual Congress. 2002:P2739.
-
- Villa J, Kuna P, Egner J, Brander R. A 6‐month comparison of the safety profiles of formoterol (Oxis® turbuhaler®) as needed and terbutaline (Bricanyl® turbuhaler®) as needed in asthmatic children on anti‐inflammatory medication. American Journal of Respiratory and Critical Care Medicine 2002;165(8 Suppl):A746.
References to studies excluded from this review
Bisgaard 2005 {published data only}
-
- Bisgaard H, Hultquist C. Efficacy and safety of budesonide/formoterol (Symbicort) Turbuhaler®as Single Therapy in patients with mild‐moderate asthma. Comparison with Symbicort Turbuhaler and Pulmicort® Turbuhaler as maintenance therapy, both complemented with Bricanyl® Turbuhaler (STAY). AstraZeneca Study Report 2005, issue SD‐039‐0673.
Boskovska 2001 {published data only}
-
- Boskovska MI, Gerovski BD, Dimitrovski TM, Arbutina SD, Jovkovsa Kaeva BM. Efficacy of long‐term formoterol therapy vs as‐needed salbutamol use in patients with moderate asthma. European Respiratory Journal 2001;18(Suppl 33):426s.
Cheung 2006 {published data only}
-
- Cheung D, Klink HCJ, Aalbers R. Improved lung function and symptom control with formoterol on demand in asthma. European Respiratory Journal 2006;27(3):504‐10. - PubMed
-
- Cheung D, Klink RCJ, Aalbers R. Improved asthma control with formoterol "as needed" compared to salbutamol in patients with asthma. American Thoracic Society 99th International Conference. 2003:B036 Poster H73.
-
- Klink HCJ, Cheung D, Aalbers R. Formoterol as relief medication is increasingly more effective than salbutamol in patients with increasing need for reliever therapy. European Respiratory Journal 2003;22(Suppl 45):P1572.
Kesten 1991 {published data only}
-
- Kesten S, Chapman KR, Broder I, Cartier A, Hyland RH, Knight A, et al. A three‐month comparison of twice daily inhaled formoterol versus four times daily inhaled albuterol in the management of stable asthma. American Review of Respiratory Disease 1991:622‐5. - PubMed
O'Connor 2000 {published data only}
-
- O'Connor B, McSorley L, Turbitt M. Does treatment with eformoterol turbohaler® prn allow a reduction in the number of inhalers used to treat moderate to severe asthma? [Abstract]. American Journal of Respiratory and Critical Care Medicine 2000;161(Suppl 3):A191.
-
- O'Connor B, McSorley LC, Turbitt ML. Use of formeterol (Oxis®) Turbuhaler ® as needed in moderate to severe asthma reduces the number of inhalers used whilst maintaining effectiveness. American Journal of Respiratory and Critical Care Medicine. 2001; Vol. 107(2):S108.
Richter 2007 {published data only}
-
- Richter K, Hartmann U, Metzenauer P, Magnussen H. Randomised trial comparing as‐needed versus regular treatment with formoterol in patients with persistent asthma. Respiratory Medicine 2007;101(3):467‐75. - PubMed
Additional references
Adams 2008
AstraZeneca Briefing Document
-
- AstraZeneca Briefing Materials: Review of the Benefits and Risks of Formoterol‐containing Products. www.fda.gov/ohrms/dockets/ac/08/briefing/2008‐4398b1‐03‐AstraZeneca.pdf 3 November 2008.
Barnes 2007
Bisgaard 2003
-
- Bisgaard H. Effect of long‐acting beta2 agonists on exacerbation rates of asthma in children. Pediatric Pulmonology 2003;36(5):391‐8. - PubMed
BNF
-
- British National Formulary 59. http://bnf.org/bnf/bnf/current/index.htm (accessed 14 May 2010).
BTS/SIGN 2008
-
- British Thoracic Society. British Guidelines on Asthma Management. Thorax 2008; Vol. 63, issue Suppl 1.
Cates 2008
Cates 2008a
Cates 2009
Cates 2009a
Cates 2009b
-
- Cates CJ, Lasserson TJ, Jaeschke R. Regular treatment with formoterol and inhaled steroids for chronic asthma: serious adverse events. Cochrane Database of Systematic Reviews 2009, Issue 2. [10.1002/14651858. CD006924.pub2] - PubMed
Delea 2008
-
- Delea TE, Hagiwara M, Stanford RH, Stempel DA. Effects of fluticasone propionate/salmeterol combination on asthma‐related health care resource utilization and costs and adherence in children and adults with asthma. Clinical Therapeutics 2008; Vol. 30, issue 3:560‐71. - PubMed
FDA website
-
- Information for Healthcare Professionals ‐ Formoterol fumarate (marketed as Foradil). http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPa... (accessed 11 May 2010).
Higgins 2003
Higgins 2008
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008] Available from: www.cochrane‐handbook.org. The Cochrane Collaboration, 2008.
Lipworth 2007
-
- Lipworth BJ, Jackson C. A SMART choice for primary care asthma therapy?. http://www.bmj.com/cgi/eletters/335/7618/513#178078 13 October 2007.
Lötvall 2008
-
- Lötvall Jan, Ankerst Jaro. Long duration of airway but not systemic effects of inhaled formoterol in asthmatic patients. Respiratory Medicine 2008;102(3):449‐56. - PubMed
Miranda 2003
-
- Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. Journal of Allergy and Clinical Immunology 2003;113(1):101‐8. - PubMed
Palmqvist 2001
-
- Palmqvist M, Arvidsson P, Beckman O, Peterson S, Lotvall J. Onset of bronchodilation of budesonide/formoterol vs. salmeterol/fluticasone in single inhalers. Pulmonary Pharmacology & Therapeutics 2001;14(1):29‐34. - PubMed
Pavord 2009
-
- Pavord ID, Jeffery PK, Qiu Y, Zhu J, Parker D, Carlsheimer A, et al. Airway inflammation in patients with asthma with high‐fixed or low‐fixed plus as‐needed budesonide/formoterol. Journal of Allergy and Clinical Immunology. 2009;123(5):1083‐9. - PubMed
RevMan 2008 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Rodrigo 2010
-
- Rodrigo Gustavo J. Increased risk of asthma death with salmeterol monotherapy compared with placebo, but not with salmeterol plus inhaled corticosteroids compared with inhaled corticosteroids alone. Evidence Based Medicine 2010;15(2):37‐8. - PubMed
Sovani 2008
Walters 2007
Wijesinghe 2009
-
- Wijesinghe M, Weatherall M, Perrin K, Harwood M, Beasley R. Risk of mortality associated with formoterol: a systematic review and meta‐analysis. Postgraduate Medical Journal 2009;34(4):803‐11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

