Prevention of diabetic nephropathy in Ins2(+/)⁻(AkitaJ) mice by the mitochondria-targeted therapy MitoQ
- PMID: 20825366
- PMCID: PMC2973231
- DOI: 10.1042/BJ20100308
Prevention of diabetic nephropathy in Ins2(+/)⁻(AkitaJ) mice by the mitochondria-targeted therapy MitoQ
Abstract
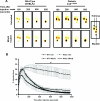
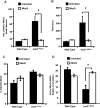
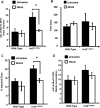
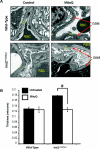
Mitochondrial production of ROS (reactive oxygen species) is thought to be associated with the cellular damage resulting from chronic exposure to high glucose in long-term diabetic patients. We hypothesized that a mitochondria-targeted antioxidant would prevent kidney damage in the Ins2(+/)⁻(AkitaJ) mouse model (Akita mice) of Type 1 diabetes. To test this we orally administered a mitochondria-targeted ubiquinone (MitoQ) over a 12-week period and assessed tubular and glomerular function. Fibrosis and pro-fibrotic signalling pathways were determined by immunohistochemical analysis, and mitochondria were isolated from the kidney for functional assessment. MitoQ treatment improved tubular and glomerular function in the Ins2(+/)⁻(AkitaJ) mice. MitoQ did not have a significant effect on plasma creatinine levels, but decreased urinary albumin levels to the same level as non-diabetic controls. Consistent with previous studies, renal mitochondrial function showed no significant change between any of the diabetic or wild-type groups. Importantly, interstitial fibrosis and glomerular damage were significantly reduced in the treated animals. The pro-fibrotic transcription factors phospho-Smad2/3 and β-catenin showed a nuclear accumulation in the Ins2(+/)⁻(AkitaJ) mice, which was prevented by MitoQ treatment. These results support the hypothesis that mitochondrially targeted therapies may be beneficial in the treatment of diabetic nephropathy. They also highlight a relatively unexplored aspect of mitochondrial ROS signalling in the control of fibrosis.
Figures





References
-
- US Renal Data System. Annual Data Report. Bethesda, MD: The National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2008.
-
- Kanwar Y. S., Wada J., Sun L., Xie P., Wallner E. I., Chen S., Chugh S., Danesh F. R. Diabetic nephropathy: mechanisms of renal disease progression. J. Exp. Med. 2008;233:4–11. - PubMed
-
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. - PubMed
-
- Green K., Brand M. D., Murphy M. P. Prevention of mitochondrial oxidative damage as a therapeutic strategy in diabetes. Diabetes. 2004;53(Suppl. 1):S110–S118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

