Cilostazol prevents amyloid β peptide(25-35)-induced memory impairment and oxidative stress in mice
- PMID: 20825411
- PMCID: PMC3010591
- DOI: 10.1111/j.1476-5381.2010.01014.x
Cilostazol prevents amyloid β peptide(25-35)-induced memory impairment and oxidative stress in mice
Abstract
Background and purpose: Cilostazol may be effective in dementia associated with a cerebral ischaemia. In this study, we examined whether it exerts beneficial effects on learning and/or memory impairment induced by Aβ(25-35) in mice, and compared its effects with those of aspirin.
Experimental approach: Aβ(25-35) (9 nmol) was administered to mice i.c.v. Learning and memory behaviour were evaluated by measuring spontaneous alternation in a Y-maze and a step-down type passive avoidance test, on the 5th and 8th days after injection respectively. Levels of lipid peroxidation (malondialdehyde) and cytokines in the frontal cortex and hippocampus were measured 2, 3, 5 and 7 days after the Aβ(25-35) injection. The effects of repeated administration of cilostazol and aspirin (both at 30 and 100 mg·kg(-1), p.o.) on any changes induced by Aβ(25-35) were evaluated.
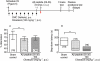
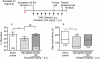
Key results: Repeated administration of cilostazol significantly attenuated the impairment of spontaneous alternation and the shortened step-down latency induced by Aβ(25-35) . Aspirin did not show any beneficial effect. A significant increase in the levels of malondialdehyde (MDA) and IL-1β (only measured in hippocampus) was observed 2, 3 and 5 days after the Aβ(25-35) injection in the frontal cortex and hippocampus. Repeated administration of cilostazol (100 mg·kg(-1)) completely prevented the increase in MDA levels but failed to antagonize the increase in the expression of IL-1β induced by Aβ(25-35).
Conclusions and implications: These results suggest that the protective effect of cilostazol on Aβ(25-35)-induced memory impairment may be related to oxidative stress in the frontal cortex and the hippocampus.
© 2010 The Authors. British Journal of Pharmacology © 2010 The British Pharmacological Society.
Figures






Similar articles
-
Silibinin prevents amyloid beta peptide-induced memory impairment and oxidative stress in mice.Br J Pharmacol. 2009 Aug;157(7):1270-7. doi: 10.1111/j.1476-5381.2009.00295.x. Epub 2009 Jun 22. Br J Pharmacol. 2009. PMID: 19552690 Free PMC article.
-
Rhizophora mucronata attenuates beta-amyloid induced cognitive dysfunction, oxidative stress and cholinergic deficit in Alzheimer's disease animal model.Metab Brain Dis. 2016 Aug;31(4):937-49. doi: 10.1007/s11011-016-9831-0. Epub 2016 May 17. Metab Brain Dis. 2016. PMID: 27188290
-
Genistein ameliorates learning and memory deficits in amyloid β(1-40) rat model of Alzheimer's disease.Neurobiol Learn Mem. 2011 Mar;95(3):270-6. doi: 10.1016/j.nlm.2010.12.001. Epub 2010 Dec 7. Neurobiol Learn Mem. 2011. PMID: 21144907
-
Jujuboside A, a neuroprotective agent from semen Ziziphi Spinosae ameliorates behavioral disorders of the dementia mouse model induced by Aβ 1-42.Eur J Pharmacol. 2014 Sep 5;738:206-13. doi: 10.1016/j.ejphar.2014.05.041. Epub 2014 Jun 2. Eur J Pharmacol. 2014. PMID: 24886882
-
Trigonelline protects hippocampus against intracerebral Aβ(1-40) as a model of Alzheimer's disease in the rat: insights into underlying mechanisms.Metab Brain Dis. 2019 Feb;34(1):191-201. doi: 10.1007/s11011-018-0338-8. Epub 2018 Nov 12. Metab Brain Dis. 2019. PMID: 30421246
Cited by
-
Drug repurposing for neurodegenerative diseases using Zebrafish behavioral profiles.Biomed Pharmacother. 2024 Feb;171:116096. doi: 10.1016/j.biopha.2023.116096. Epub 2024 Jan 6. Biomed Pharmacother. 2024. PMID: 38185043 Free PMC article.
-
New therapeutic approaches for Alzheimer's disease and cerebral amyloid angiopathy.Front Aging Neurosci. 2014 Oct 20;6:290. doi: 10.3389/fnagi.2014.00290. eCollection 2014. Front Aging Neurosci. 2014. PMID: 25368578 Free PMC article. Review.
-
Phosphodiesterase-5 Inhibitors: Action on the Signaling Pathways of Neuroinflammation, Neurodegeneration, and Cognition.Mediators Inflamm. 2015;2015:940207. doi: 10.1155/2015/940207. Epub 2015 Dec 3. Mediators Inflamm. 2015. PMID: 26770022 Free PMC article. Review.
-
Neuroprotective Effect of 2-Aminoethoxydiphenyl Borate (2-APB) in Amyloid β-Induced Memory Dysfunction: A Mechanistic Study.Cell Mol Neurobiol. 2022 May;42(4):1211-1223. doi: 10.1007/s10571-020-01012-z. Epub 2020 Nov 21. Cell Mol Neurobiol. 2022. PMID: 33219878 Free PMC article.
-
Neuroprotective Effect of Ligustilide through Induction of α-Secretase Processing of Both APP and Klotho in a Mouse Model of Alzheimer's Disease.Front Aging Neurosci. 2017 Nov 2;9:353. doi: 10.3389/fnagi.2017.00353. eCollection 2017. Front Aging Neurosci. 2017. PMID: 29163135 Free PMC article.
References
-
- Alkam T, Nitta A, Mizoguchi H, Itoh A, Nabeshima T. A natural scavenger of peroxynitrites, rosmarinic acid, protects against impairment of memory induced by Abeta(25-35) Behav Brain Res. 2007;180:139–145. - PubMed
-
- Alkam T, Nitta A, Mizoguchi H, Itoh A, Murai R, Nagai T, et al. The extensive nitration of neurofilament light chain in the hippocampus is associated with the cognitive impairment induced by amyloid beta in mice. J Pharmacol Exp Ther. 2008;327:137–147. - PubMed
-
- Arai H, Takahashi T. A combination therapy of donepezil and cilostazol for patients with moderate Alzheimer disease: pilot follow-up study. Am J Geriatr Psychiatry. 2009;17:353–354. - PubMed
-
- Choi JM, Shin HK, Kim KY, Lee JH, Hong KW. Neuroprotective effect of cilostazol against focal cerebral ischemia via antiapoptotic action in rats. J Pharmacol Exp Ther. 2002;300:787–793. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

