Role of cytotoxic protease granzyme-b in neuronal degeneration during human stroke
- PMID: 20825413
- PMCID: PMC8094313
- DOI: 10.1111/j.1750-3639.2010.00426.x
Role of cytotoxic protease granzyme-b in neuronal degeneration during human stroke
Abstract
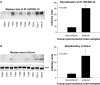
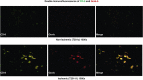
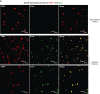
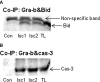
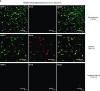
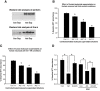
Infiltration of leukocytes into post-ischemic cerebrum is a well-described phenomenon in stroke injury. Because CD-8(+) T-lymphocytes secrete cytotoxic proteases, including granzyme-b (Gra-b) that exacerbates post-ischemic brain damage, we investigated roles of Gra-b in human stroke. To study the role of Gra-b in stroke, ischemic and non-ischemic tissues (from post-mortem stroke patients) were analyzed using immunoblotting, co-immunoprecipitation, terminal deoxy uridine nick end labeling (TUNEL) and Annexin-V immunostaining, and in vitro neuron survival assays. Activated CG-SH cells and supernatants were used to model leukocyte-dependent injury. Non-ischemic brain tissues were used as non-pathological controls. Non-activated CG-SH cells and supernatants were used as controls for in vitro experiments. Human stroke (ischemic) samples contained significantly higher levels of Gra-b and interferon-gamma inducible protein-10 (IP-10/CXCL10) than non-ischemic controls. In stroke, poly (ADP-ribose) polymerase-1 and heat shock protein-70 were cleaved to canonical proteolytic "signature" fragments by Gra-b. Gra-b was also found to bind to Bid and caspase-3. Gra-b also co-localized with Annexin-V(+) /TUNEL(+) in degenerating neurons. Importantly, Gra-b inhibition protected both normal and ischemia-reperfused neurons against in vitro neurotoxicity mediated by activated CG-SH cells and supernatants. These results suggest that increased leukocyte infiltration and elevated Gra-b levels in the post-stroke brain can induce contact-dependent and independent post-ischemic neuronal death to aggravate stroke injury.
© 2010 The Authors; Brain Pathology © 2010 International Society of Neuropathology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Sherman DG, Bes A, Easton JD, Hacke W, Kaste M, Polmar SH et al (2001) Use of anti‐ICAM‐1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology 57:1428–1434. - PubMed
-
- Alexander JS, Dayton T, Davis C, Hill S, Jackson TH, Blaschuk O et al (1998) Activated T‐lymphocytes express occludin, a component of tight junctions. Inflammation 22:573–582. - PubMed
-
- Alimonti JB, Shi L, Baijal PK, Greenberg AH (2001) Granzyme B induces BID‐mediated cytochrome c release and mitochondrial permeability transition. J Biol Chem 276:6974–6982. - PubMed
-
- Amantea D, Nappi G, Bernardi G, Bagetta G, Corasaniti MT (2009) Post‐ischemic brain damage: pathophysiology and role of inflammatory mediators. FEBS J 276:13–26. - PubMed
-
- Andersen MH, Becker JC, Thor SP (2005) The antiapoptotic member of the Bcl‐2 family Mcl‐1 is a CTL target in cancer patients. Leukemia 19:484–485. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

