Bone morphogenetic protein signaling regulates gastric epithelial cell development and proliferation in mice
- PMID: 20826155
- PMCID: PMC3039550
- DOI: 10.1053/j.gastro.2010.08.052
Bone morphogenetic protein signaling regulates gastric epithelial cell development and proliferation in mice
Abstract
Background & aims: We investigated the role of bone morphogenetic protein (BMP) signaling in the regulation of gastric epithelial cell growth and differentiation by generating transgenic mice that express the BMP inhibitor noggin in the stomach.
Methods: The promoter of the mouse H+/K+-ATPase β-subunit gene, which is specifically expressed in parietal cells, was used to regulate expression of noggin in the gastric epithelium of mice. The transgenic mice were analyzed for noggin expression, tissue morphology, cellular composition of the gastric mucosa, gastric acid content, and plasma levels of gastrin. Tissues were analyzed by immunohistochemical, quantitative real-time polymerase chain reaction, immunoblot, microtitration, and radioimmunoassay analyses.
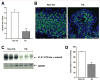
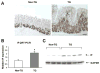
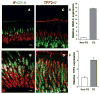
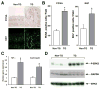
Results: In the stomachs of the transgenic mice, phosphorylation of Smad 1, 5, and 8 decreased, indicating inhibition of BMP signaling. Mucosa were of increased height, with dilated glands, cystic structures, reduced numbers of parietal cells, and increased numbers of cells that coexpressed intrinsic factor, trefoil factor 2, and Griffonia (Bandeiraea) simplicifolia lectin II, compared with wild-type mice. In the transgenic mice, levels of the H+/K+-ATPase α-subunit protein and messenger RNA were reduced, whereas those of intrinsic factor increased. The transgenic mice were hypochloridric and had an increased number of Ki67- and proliferating cell nuclear antigen-positive cells; increased levels of plasma gastrin; increased expression of transforming growth factor-α, amphiregulin, and gastrin; and activation of extracellular signal-regulated kinase 2.
Conclusions: Inhibiting BMP signaling in the stomachs of mice by expression of noggin causes loss of parietal cells, development of transitional cells that express markers of mucus neck and zymogenic lineages, and activation of proliferation. BMPs are therefore important regulators of gastric epithelial cell homeostasis.
Copyright © 2010 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures



Comment in
-
Morphogens and the parietal cell: shaping up acid secretion.Gastroenterology. 2010 Dec;139(6):1830-3. doi: 10.1053/j.gastro.2010.10.035. Epub 2010 Oct 26. Gastroenterology. 2010. PMID: 21029799 No abstract available.
References
-
- Winnier G, Blessing M, Labosky PA, et al. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. - PubMed
-
- Dunn NR, Winnier GE, Hargett LK, et al. Haploinsufficient phenotypes in Bmp4 heterozygous null mice and modification by mutations in Gli3 and Alx4. Dev Biol. 1997;188:235–247. - PubMed
-
- Kawabata M, Imamura T, Miyazono K. Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev. 1998;9:49–61. - PubMed
-
- van den Brink GR, Hardwick JC, Tytgat GN, et al. Sonic hedgehog regulates gastric gland morphogenesis in man and mouse. Gastroenterology. 2001;121:317–328. - PubMed
-
- Madison BB, Braunstein K, Kuizon E, et al. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development. 2005;132:279–289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

