E3 ubiquitin protein ligase, E6-associated protein (E6-AP) regulates PI3K-Akt signaling and prostate cell growth
- PMID: 20826237
- PMCID: PMC3031754
- DOI: 10.1016/j.bbagrm.2010.08.011
E3 ubiquitin protein ligase, E6-associated protein (E6-AP) regulates PI3K-Akt signaling and prostate cell growth
Abstract
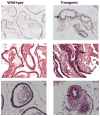
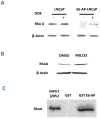
This study elucidates the role of E6-associated protein, E6-AP (a dual function steroid hormone receptor coactivator and ubiquitin-protein ligase) in the regulation of PI3K-Akt signaling pathway, prostate gland growth and proliferation. Here, we report the generation of transgenic mice and prostate cancer cell line, LNCaP cells that overexpress E6-AP protein. Using these models we show that the levels of total Akt and phosphorylated Akt (active Akt) are increased in E6-AP overexpressing prostate gland and LNCaP cells suggesting that E6-AP regulates the PI3K-Akt signaling pathway. The prostate glands in our transgenic mice are ~20% larger and produce preneoplastic lesions at the age of 18 months. Our data also suggest that E6-AP modulates PI3K-Akt signaling pathway by both androgen-independent and -dependent mechanisms. In the androgen-independent mechanism, E6-AP modulates PI3K-Akt signaling by regulating the protein levels of RhoA, a small GTPase, which is a negative regulator of the Akt signaling pathway. Further, we show that E6-AP, a known coactivator of AR, amplifies the androgen-dependent activation of PI3K-Akt signaling pathway. In addition, we show that stable overexpression of E6-AP in prostate cancer cells results in increased cell size and proliferation. Overall our data suggests that E6-AP regulates both the positive and negative modulators of the PI3K-Akt pathway in prostate cells which results in increased prostate cell growth, proliferation and decreased apoptosis.This article is part of a Special Issue entitled The 26S Proteasome: When degradation is just not enough!
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures




Similar articles
-
Multifunction steroid receptor coactivator, E6-associated protein, is involved in development of the prostate gland.Mol Endocrinol. 2006 Mar;20(3):544-59. doi: 10.1210/me.2005-0110. Epub 2005 Oct 27. Mol Endocrinol. 2006. PMID: 16254014
-
Impact of E6-associated protein on the proliferation and invasion of prostate cancer cells in bone metastasis.Int J Clin Exp Pathol. 2015 Jun 1;8(6):6571-5. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26261538 Free PMC article.
-
Overexpression of ligase defective E6-associated protein, E6-AP, results in mammary tumorigenesis.Breast Cancer Res Treat. 2012 Feb;132(1):97-108. doi: 10.1007/s10549-011-1567-2. Epub 2011 May 8. Breast Cancer Res Treat. 2012. PMID: 21553290
-
The PI3K-AKT-mTOR Pathway and Prostate Cancer: At the Crossroads of AR, MAPK, and WNT Signaling.Int J Mol Sci. 2020 Jun 25;21(12):4507. doi: 10.3390/ijms21124507. Int J Mol Sci. 2020. PMID: 32630372 Free PMC article. Review.
-
Interplay Among PI3K/AKT, PTEN/FOXO and AR Signaling in Prostate Cancer.Adv Exp Med Biol. 2019;1210:319-331. doi: 10.1007/978-3-030-32656-2_14. Adv Exp Med Biol. 2019. PMID: 31900915 Review.
Cited by
-
E6AP promotes prostate cancer by reducing p27 expression.Oncotarget. 2017 Jun 27;8(26):42939-42948. doi: 10.18632/oncotarget.17224. Oncotarget. 2017. PMID: 28477016 Free PMC article.
-
New Strategies to Direct Therapeutic Targeting of PML to Treat Cancers.Front Oncol. 2013 May 17;3:124. doi: 10.3389/fonc.2013.00124. eCollection 2013. Front Oncol. 2013. PMID: 23730625 Free PMC article.
-
Effect of dexamethasone and testosterone treatment on the regulation of insulin-degrading enzyme and cellular changes in ventral rat prostate after castration.Int J Exp Pathol. 2011 Aug;92(4):272-80. doi: 10.1111/j.1365-2613.2011.00772.x. Epub 2011 Apr 21. Int J Exp Pathol. 2011. PMID: 21507087 Free PMC article.
-
Post-Translational Modifications That Drive Prostate Cancer Progression.Biomolecules. 2021 Feb 9;11(2):247. doi: 10.3390/biom11020247. Biomolecules. 2021. PMID: 33572160 Free PMC article. Review.
-
SOX11 exacerbates ferroptosis to reduce lenvatinib resistance in liver cancer cells by promoting ubiquitination degradation of SREBF1 through upregulating UBE3A.Mol Cell Biochem. 2025 Jul;480(7):4119-4134. doi: 10.1007/s11010-025-05218-x. Epub 2025 Mar 1. Mol Cell Biochem. 2025. PMID: 40025300
References
-
- Khan OY, Fu G, Ismail A, Srinivasan S, Cao X, Tu Y, Lu S, Nawaz Z. Multifunction steroid receptor coactivator, E6-associated protein, is involved in development of the prostate gland. Mol Endocrinol. 2006;20:544–559. - PubMed
-
- Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75:495–505. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

