Differences in gamma frequencies across visual cortex restrict their possible use in computation
- PMID: 20826318
- PMCID: PMC3001273
- DOI: 10.1016/j.neuron.2010.08.004
Differences in gamma frequencies across visual cortex restrict their possible use in computation
Abstract
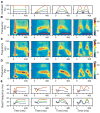
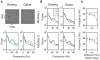
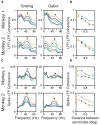
Neuronal oscillations in the gamma band (30-80 Hz) have been suggested to play a central role in feature binding or establishing channels for neural communication. For these functions, the gamma rhythm frequency must be consistent across neural assemblies encoding the features of a stimulus. Here we test the dependence of gamma frequency on stimulus contrast in V1 cortex of awake behaving macaques and show that gamma frequency increases monotonically with contrast. Changes in stimulus contrast over time leads to a reliable gamma frequency modulation on a fast timescale. Further, large stimuli whose contrast varies across space generate gamma rhythms at significantly different frequencies in simultaneously recorded neuronal assemblies separated by as little as 400 microm, making the gamma rhythm a poor candidate for binding or communication, at least in V1. Instead, our results suggest that the gamma rhythm arises from local interactions between excitation and inhibition.
2010 Elsevier Inc. All rights reserved.
Figures


References
-
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. - PubMed
-
- Berens P, Velasco MJ. MPI Technical Report No 184. 2009.
-
- Brunel N, Wang XJ. What determines the frequency of fast network oscillations with irregular neural discharges? I. Synaptic dynamics and excitation-inhibition balance. J Neurophysiol. 2003;90:415–430. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

