Reduced striatal dopamine underlies the attention system dysfunction in neurofibromatosis-1 mutant mice
- PMID: 20826448
- PMCID: PMC2957316
- DOI: 10.1093/hmg/ddq382
Reduced striatal dopamine underlies the attention system dysfunction in neurofibromatosis-1 mutant mice
Abstract
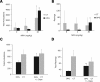
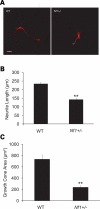
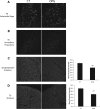
Learning and behavioral abnormalities are among the most common clinical problems in children with the neurofibromatosis-1 (NF1) inherited cancer syndrome. Recent studies using Nf1 genetically engineered mice (GEM) have been instructive for partly elucidating the cellular and molecular defects underlying these cognitive deficits; however, no current model has shed light on the more frequently encountered attention system abnormalities seen in children with NF1. Using an Nf1 optic glioma (OPG) GEM model, we report novel defects in non-selective and selective attention without an accompanying hyperactivity phenotype. Specifically, Nf1 OPG mice exhibit reduced rearing in response to novel objects and environmental stimuli. Similar to children with NF1, the attention system dysfunction in these mice is reversed by treatment with methylphenidate (MPH), suggesting a defect in brain catecholamine homeostasis. We further demonstrate that this attention system abnormality is the consequence of reduced dopamine (DA) levels in the striatum, which is normalized following either MPH or l-dopa administration. The reduction in striatal DA levels in Nf1 OPG mice is associated with reduced striatal expression of tyrosine hydroxylase, the rate-limited enzyme in DA synthesis, without any associated dopaminergic cell loss in the substantia nigra. Moreover, we demonstrate a cell-autonomous defect in Nf1+/- dopaminergic neuron growth cone areas and neurite extension in vitro, which results in decreased dopaminergic cell projections to the striatum in Nf1 OPG mice in vivo. Collectively, these data establish abnormal DA homeostasis as the primary biochemical defect underlying the attention system dysfunction in Nf1 GEM relevant to children with NF1.
Figures




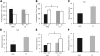

Similar articles
-
Elucidating the impact of neurofibromatosis-1 germline mutations on neurofibromin function and dopamine-based learning.Hum Mol Genet. 2015 Jun 15;24(12):3518-28. doi: 10.1093/hmg/ddv103. Epub 2015 Mar 18. Hum Mol Genet. 2015. PMID: 25788518 Free PMC article.
-
Sex Is a major determinant of neuronal dysfunction in neurofibromatosis type 1.Ann Neurol. 2014 Feb;75(2):309-16. doi: 10.1002/ana.24093. Epub 2014 Feb 6. Ann Neurol. 2014. PMID: 24375753 Free PMC article.
-
Neurofibromatosis-1 heterozygosity impairs CNS neuronal morphology in a cAMP/PKA/ROCK-dependent manner.Mol Cell Neurosci. 2012 Jan;49(1):13-22. doi: 10.1016/j.mcn.2011.08.008. Epub 2011 Aug 26. Mol Cell Neurosci. 2012. PMID: 21903164 Free PMC article.
-
Insights into optic pathway glioma vision loss from mouse models of neurofibromatosis type 1.J Neurosci Res. 2019 Jan;97(1):45-56. doi: 10.1002/jnr.24250. Epub 2018 Apr 28. J Neurosci Res. 2019. PMID: 29704429 Free PMC article. Review.
-
Neurofibromin in the brain.J Child Neurol. 2002 Aug;17(8):592-601; discussion 602-4, 646-51. doi: 10.1177/088307380201700809. J Child Neurol. 2002. PMID: 12403558 Review.
Cited by
-
Dopamine deficiency underlies learning deficits in neurofibromatosis-1 mice.Ann Neurol. 2013 Feb;73(2):309-15. doi: 10.1002/ana.23793. Epub 2012 Dec 7. Ann Neurol. 2013. PMID: 23225063 Free PMC article.
-
Steady-state visual evoked potentials in children with neurofibromatosis type 1: associations with behavioral rating scales and impact of psychostimulant medication.J Neurodev Disord. 2022 Jul 22;14(1):42. doi: 10.1186/s11689-022-09452-y. J Neurodev Disord. 2022. PMID: 35869419 Free PMC article.
-
Autism-associated Nf1 deficiency disrupts corticocortical and corticostriatal functional connectivity in human and mouse.Neurobiol Dis. 2019 Oct;130:104479. doi: 10.1016/j.nbd.2019.104479. Epub 2019 May 22. Neurobiol Dis. 2019. PMID: 31128207 Free PMC article.
-
RAS and beyond: the many faces of the neurofibromatosis type 1 protein.Dis Model Mech. 2022 Feb 1;15(2):dmm049362. doi: 10.1242/dmm.049362. Epub 2022 Feb 21. Dis Model Mech. 2022. PMID: 35188187 Free PMC article.
-
Oligodendrocyte Nf1 Controls Aberrant Notch Activation and Regulates Myelin Structure and Behavior.Cell Rep. 2017 Apr 18;19(3):545-557. doi: 10.1016/j.celrep.2017.03.073. Cell Rep. 2017. PMID: 28423318 Free PMC article.
References
-
- Coude F.X., Mignot C., Lyonnet S., Munnich A. Academic impairment is the most frequent complication of neurofibromatosis type-1 (NF1) in children. Behav. Genet. 2006;36:660–664. - PubMed
-
- Descheemaeker M.J., Ghesquiere P., Symons H., Fryns J.P., Legius E. Behavioural, academic and neuropsychological profile of normally gifted Neurofibromatosis type 1 children. J. Intellect. Disabil. Res. 2005;49:33–46. - PubMed
-
- Dilts C.V., Carey J.C., Kircher J.C., Hoffman R.O., Creel D., Ward K., Clark E., Leonard C.O. Children and adolescents with neurofibromatosis 1: a behavioral phenotype. J. Dev. Behav. Pediatr. 1996;17:229–239. - PubMed
-
- Hyman S.L., Shores A., North K.N. The nature and frequency of cognitive deficits in children with neurofibromatosis type 1. Neurology. 2005;65:1037–1044. - PubMed
-
- North K.N., Riccardi V., Samango-Sprouse C., Ferner R., Moore B., Legius E., Ratner N., Denckla M.B. Cognitive function and academic performance in neurofibromatosis 1: consensus statement from the NF1 Cognitive Disorders Task Force. Neurology. 1997;48:1121–1127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

