The RAV1 transcription factor positively regulates leaf senescence in Arabidopsis
- PMID: 20826506
- PMCID: PMC2935868
- DOI: 10.1093/jxb/erq206
The RAV1 transcription factor positively regulates leaf senescence in Arabidopsis
Abstract
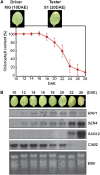
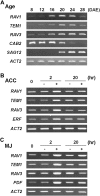
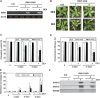
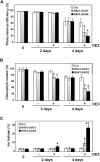
Leaf senescence is a developmentally programmed cell death process that constitutes the final step of leaf development and involves the extensive reprogramming of gene expression. Despite the importance of senescence in plants, the underlying regulatory mechanisms are not well understood. This study reports the isolation and functional analysis of RAV1, which encodes a RAV family transcription factor. Expression of RAV1 and its homologues is closely associated with leaf maturation and senescence. RAV1 mRNA increased at a later stage of leaf maturation and reached a maximal level early in senescence, but decreased again during late senescence. This profile indicates that RAV1 could play an important regulatory role in the early events of leaf senescence. Furthermore, constitutive and inducible overexpression of RAV1 caused premature leaf senescence. These data strongly suggest that RAV1 is sufficient to cause leaf senescence and it functions as a positive regulator in this process.
Figures



References
-
- Alonso JM, Stepanova AN, Leisse TJ, et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science. 2003;301:653–657. - PubMed
-
- Balazadeh S, Riaño-Pachón DM, Mueller-Roeber B. Transcription factors regulating leaf senescence in Arabidopsis thaliana. Plant Biololgy. 2008;10:63–75. - PubMed
-
- Buchanan-Wollaston V, Earl S, Harrison E, Mathas E, Navabpour S, Page T, Pink D. The molecular analysis of leaf senescence: a genomics approach. Plant Biotechnology Journal. 2003;1:3–22. - PubMed
-
- Buchanan-Wollaston V, Page T, Harrison E, et al. Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. The Plant Journal. 2005;42:567–585. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

