Epidermal growth factor receptor kinase activity is required for gap junction closure and for part of the decrease in ovarian follicle cGMP in response to LH
- PMID: 20826538
- PMCID: PMC3119707
- DOI: 10.1530/REP-10-0288
Epidermal growth factor receptor kinase activity is required for gap junction closure and for part of the decrease in ovarian follicle cGMP in response to LH
Abstract
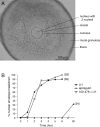
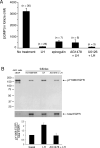
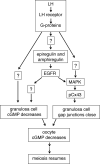
The meiotic cell cycle in mouse oocytes is arrested in prophase, and then restarted when LH acts on the surrounding granulosa cells. The granulosa cells keep meiosis arrested by providing a source of cGMP that diffuses into the oocyte through gap junctions, and LH restarts the cell cycle by closing the junctions and by decreasing granulosa cell cGMP, thus lowering oocyte cGMP. Epidermal growth factor receptor (EGFR) activation is an essential step in triggering LH-induced meiotic resumption, but its relationship to the cGMP decrease in the follicle is incompletely understood, and its possible function in causing gap junction closure has not been investigated. Here, we use EGFR agonists (epiregulin and amphiregulin) and an EGFR kinase inhibitor (AG1478) to study the function of the EGFR in the signaling pathways leading to the release of oocytes from prophase arrest. Our results indicate that the EGFR kinase contributes to LH-induced meiotic resumption in two different ways. First, it is required for gap junction closure. Second, it is required for an essential component of the decrease in follicle cGMP. Our data show that the EGFR kinase-dependent component of the cGMP decrease is required for LH-induced meiotic resumption, but they also indicate that an as yet unidentified pathway accounts for a large part of the cGMP decrease.
Figures


References
-
- Ashkenazi H, Cao X, Motola S, Popliker M, Conti M, Tsafriri A. Epidermal growth factor family members: endogenous mediators of the ovulatory response. Endocrinology. 2005;146:77–84. - PubMed
-
- Dekel N, Sherizly I. Epidermal growth factor induces maturation of rat follicle-enclosed oocytes. Endocrinology. 1985;116:406–409. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

