Muramyl dipeptide synergizes with Staphylococcus aureus lipoteichoic acid to recruit neutrophils in the mammary gland and to stimulate mammary epithelial cells
- PMID: 20826612
- PMCID: PMC2976092
- DOI: 10.1128/CVI.00268-10
Muramyl dipeptide synergizes with Staphylococcus aureus lipoteichoic acid to recruit neutrophils in the mammary gland and to stimulate mammary epithelial cells
Abstract
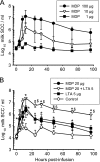
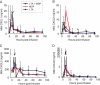
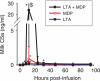
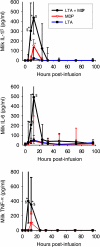
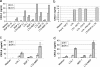
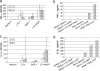
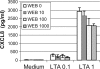
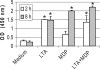
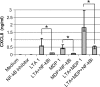
Staphylococcus aureus, a major pathogen for the mammary gland of dairy ruminants, elicits the recruitment of neutrophils into milk during mastitis, but the mechanisms are incompletely understood. We investigated the response of the bovine mammary gland to muramyl dipeptide (MDP), an elementary constituent of the bacterial peptidoglycan, alone or in combination with lipoteichoic acid (LTA), another staphylococcal microbial-associated molecular pattern (MAMP). MDP induced a prompt and marked influx of neutrophils in milk, and its combination with LTA elicited a more intense and prolonged influx than the responses to either stimulus alone. The concentrations of several chemoattractants for neutrophils (CXCL1, CXCL2, CXCL3, CXCL8, and C5a) increased in milk after challenge, and the highest increases followed challenge with the combination of MDP and LTA. MDP and LTA were also synergistic in inducing in vitro chemokine production by bovine mammary epithelial cells (bMEpC). Nucleotide-binding oligomerization domain 2 (NOD2), a major sensor of MDP, was expressed (mRNA) in bovine mammary tissue and by bMEpC in culture. The production of interleukin-8 (IL-8) following the stimulation of bMEpC by LTA and MDP was dependent on the activation of NF-κB. LTA-induced IL-8 production did not depend on platelet-activating factor receptor (PAFR), as the PAFR antagonist WEB2086 was without effect. In contrast, bMEpC and mammary tissue are known to express Toll-like receptor 2 (TLR2) and to respond to TLR2 agonists. Although the levels of expression of the inflammatory cytokines tumor necrosis factor alpha (TNF-α) and IL-1β were increased by LTA and MDP at the mRNA level, no protein could be detected in the bMEpC culture supernatant. The level of induction of IL-6 was low at both the mRNA and protein levels. These results indicate that MDP and LTA exert synergistic effects to induce neutrophilic inflammation in the mammary gland. These results also show that bMEpC could contribute to the inflammatory response by recognizing LTA and MDP and secreting chemokines but not proinflammatory cytokines. Overall, this study indicates that the TLR2 and NOD2 pathways could cooperate to trigger an innate immune response to S. aureus mastitis.
Figures
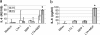

Similar articles
-
Staphylococcus aureus lipoteichoic acid triggers inflammation in the lactating bovine mammary gland.Vet Res. 2008 Sep-Oct;39(5):52. doi: 10.1051/vetres:2008034. Epub 2008 Jul 3. Vet Res. 2008. PMID: 18593548
-
Muramyl dipeptide potentiates staphylococcal lipoteichoic acid induction of cyclooxygenase-2 expression in macrophages.Microbes Infect. 2014 Feb;16(2):153-60. doi: 10.1016/j.micinf.2013.10.018. Epub 2013 Nov 7. Microbes Infect. 2014. PMID: 24211871
-
Muramyl dipeptide potentiates Staphylococcus aureus lipoteichoic acid-induced nitric oxide production via TLR2/NOD2/PAFR signaling pathways.Front Immunol. 2024 Dec 6;15:1451315. doi: 10.3389/fimmu.2024.1451315. eCollection 2024. Front Immunol. 2024. PMID: 39712020 Free PMC article.
-
Staphylococcus aureus metal acquisition in the mastitic mammary gland.Microb Pathog. 2020 Jul;144:104179. doi: 10.1016/j.micpath.2020.104179. Epub 2020 Mar 31. Microb Pathog. 2020. PMID: 32244043 Review.
-
Immunology of the Bovine Mammary Gland: Advances in Recent Years.Vet Clin North Am Food Anim Pract. 2025 Jul;41(2):137-154. doi: 10.1016/j.cvfa.2025.03.002. Epub 2025 Apr 23. Vet Clin North Am Food Anim Pract. 2025. PMID: 40274420 Review.
Cited by
-
Non-classical proIL-1beta activation during mammary gland infection is pathogen-dependent but caspase-1 independent.PLoS One. 2014 Aug 27;9(8):e105680. doi: 10.1371/journal.pone.0105680. eCollection 2014. PLoS One. 2014. PMID: 25162221 Free PMC article.
-
Immune defenses of the mammary gland epithelium of dairy ruminants.Front Immunol. 2022 Oct 21;13:1031785. doi: 10.3389/fimmu.2022.1031785. eCollection 2022. Front Immunol. 2022. PMID: 36341445 Free PMC article. Review.
-
Metformin Inhibits Lipoteichoic Acid-Induced Oxidative Stress and Inflammation Through AMPK/NRF2/NF-κB Signaling Pathway in Bovine Mammary Epithelial Cells.Front Vet Sci. 2021 Jun 28;8:661380. doi: 10.3389/fvets.2021.661380. eCollection 2021. Front Vet Sci. 2021. PMID: 34262962 Free PMC article.
-
Differential response of bovine mammary epithelial cells to Staphylococcus aureus or Escherichia coli agonists of the innate immune system.Vet Res. 2013 Jun 11;44(1):40. doi: 10.1186/1297-9716-44-40. Vet Res. 2013. PMID: 23758654 Free PMC article.
-
Extracellular milieu grossly alters pathogen-specific immune response of mammary epithelial cells.BMC Vet Res. 2015 Jul 30;11:172. doi: 10.1186/s12917-015-0489-3. BMC Vet Res. 2015. PMID: 26219462 Free PMC article.
References
-
- Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124:783-801. - PubMed
-
- Bannerman, D. D. 2009. Pathogen-dependent induction of cytokines and other soluble inflammatory mediators during intramammary infection of dairy cows. J. Anim. Sci. 87:10-25. - PubMed
-
- Bergonier, D., and X. Berthelot. 2003. New advances in epizootiology and control of ewe mastitis. Livestock Production Sci. 79:1-16.
-
- Botrel, M. A., M. Haenni, E. Morignat, P. Sulpice, J. Y. Madec, and D. Calavas. 2010. Distribution and antimicrobial resistance of clinical and subclinical mastitis pathogens in dairy cows in Rhone-Alpes, France. Foodborne Pathog. Dis. 7:479-487. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

