The role of contralesional dorsal premotor cortex after stroke as studied with concurrent TMS-fMRI
- PMID: 20826657
- PMCID: PMC3044467
- DOI: 10.1523/JNEUROSCI.5642-09.2010
The role of contralesional dorsal premotor cortex after stroke as studied with concurrent TMS-fMRI
Abstract
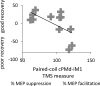
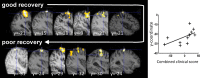
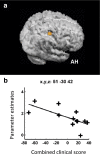
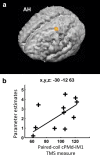
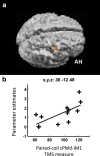
Contralesional dorsal premotor cortex (cPMd) may support residual motor function following stroke. We performed two complementary experiments to explore how cPMd might perform this role in a group of chronic human stroke patients. First, we used paired-coil transcranial magnetic stimulation (TMS) to establish the physiological influence of cPMd on ipsilesional primary motor cortex (iM1) at rest. We found that this influence became less inhibitory/more facilitatory in patients with greater clinical impairment. Second, we applied TMS over cPMd during functional magnetic resonance imaging (fMRI) in these patients to examine the causal influence of cPMd TMS on the whole network of surviving cortical motor areas in either hemisphere and whether these influences changed during affected hand movement. We confirmed that hand grip-related activation in cPMd was greater in more impaired patients. Furthermore, the peak ipsilesional sensorimotor cortex activity shifted posteriorly in more impaired patients. Critical new findings were that concurrent TMS-fMRI results correlated with the level of both clinical impairment and neurophysiological impairment (i.e., less inhibitory/more facilitatory cPMd-iM1 measure at rest as assessed with paired-coil TMS). Specifically, greater clinical and neurophysiological impairment was associated with a stronger facilitatory influence of cPMd TMS on blood oxygenation level-dependent signal in posterior parts of ipsilesional sensorimotor cortex during hand grip, corresponding to the posteriorly shifted sensorimotor activity seen in more impaired patients. cPMd TMS was not found to influence activity in other brain regions in either hemisphere. This state-dependent influence on ipsilesional sensorimotor regions may provide a mechanism by which cPMd supports recovered function after stroke.
Figures


References
-
- Amassian VE, Stewart M. Motor cortical and other cortical interneuronal networks that generate very high frequency waves. Suppl Clin Neurophysiol. 2003;56:119–142. - PubMed
-
- Andersson JL, Hutton C, Ashburner J, Turner R, Friston K. Modeling geometric deformations in EPI time series. Neuroimage. 2001;13:903–919. - PubMed
-
- Baudewig J, Paulus W, Frahm J. Artifacts caused by transcranial magnetic stimulation coils and EEG electrodes in T(2)*-weighted echo-planar imaging. Magn Reson Imaging. 2000;18:479–484. - PubMed
-
- Baudewig J, Siebner HR, Bestmann S, Tergau F, Tings T, Paulus W, Frahm J. Functional MRI of cortical activations induced by transcranial magnetic stimulation (TMS) Neuroreport. 2001;12:3543–3548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
