Tumor necrosis factor-alpha (TNF-alpha) regulates shedding of TNF-alpha receptor 1 by the metalloprotease-disintegrin ADAM8: evidence for a protease-regulated feedback loop in neuroprotection
- PMID: 20826683
- PMCID: PMC6633558
- DOI: 10.1523/JNEUROSCI.1520-10.2010
Tumor necrosis factor-alpha (TNF-alpha) regulates shedding of TNF-alpha receptor 1 by the metalloprotease-disintegrin ADAM8: evidence for a protease-regulated feedback loop in neuroprotection
Abstract
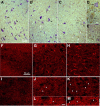
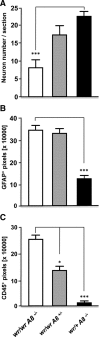
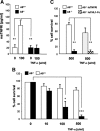
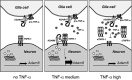
Tumor necrosis factor alpha (TNF-alpha) is a potent cytokine in neurodegenerative disorders, but its precise role in particular brain disorders is ambiguous. In motor neuron (MN) disease of the mouse, exemplified by the model wobbler (WR), TNF-alpha causes upregulation of the metalloprotease-disintegrin ADAM8 (A8) in affected brain regions, spinal cord, and brainstem. The functional role of A8 during MN degeneration in the wobbler CNS was investigated by crossing WR with A8-deficient mice: a severely aggravated neuropathology was observed for A8-deficient WR compared with WR A8(+/-) mice, judged by drastically reduced survival [7 vs 81% survival at postnatal day 50 (P50)], accelerated force loss in the forelimbs, and terminal akinesis. In vitro protease assays using soluble A8 indicated specific cleavage of a TNF-alpha receptor 1 (p55 TNF-R1) but not a TNF-R2 peptide. Cleavage of TNF-R1 was confirmed in situ, because levels of soluble TNF-R1 were increased in spinal cords of standard WR compared with wild-type mice but not in A8-deficient WR mice. In isolated primary neurons and microglia, TNF-alpha-induced TNF-R1 shedding was dependent on the A8 gene dosage. Furthermore, exogenous TNF-alpha showed higher toxicity for cultured neurons from A8-deficient than for those from wild-type mice, demonstrating that TNF-R1 shedding by A8 is neuroprotective. Our results indicate an essential role for ADAM8 in modulating TNF-alpha signaling in CNS diseases: a feedback loop integrating TNF-alpha, ADAM8, and TNF-R1 shedding as a plausible mechanism for TNF-alpha mediated neuroprotection in situ and a rationale for therapeutic intervention.
Figures




References
-
- Bigini P, Repici M, Cantarella G, Fumagalli E, Barbera S, Cagnotto A, De Luigi A, Tonelli R, Bernardini R, Borsello T, Mennini T. Recombinant human TNF-binding protein-1 (rhTBP-1) treatment delays both symptoms progression and motor neuron loss in the wobbler mouse. Neurobiol Dis. 2008;29:465–476. - PubMed
-
- Black RA. Tumor necrosis factor-alpha converting enzyme. Int J Biochem Cell Biol. 2002;34:1–5. - PubMed
-
- Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. - PubMed
-
- Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634–1635. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
