Improved endpoints for cancer immunotherapy trials
- PMID: 20826737
- PMCID: PMC2943524
- DOI: 10.1093/jnci/djq310
Improved endpoints for cancer immunotherapy trials
Abstract
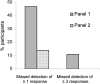
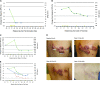
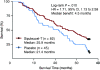
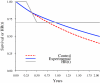
Unlike chemotherapy, which acts directly on the tumor, cancer immunotherapies exert their effects on the immune system and demonstrate new kinetics that involve building a cellular immune response, followed by changes in tumor burden or patient survival. Thus, adequate design and evaluation of some immunotherapy clinical trials require a new development paradigm that includes reconsideration of established endpoints. Between 2004 and 2009, several initiatives facilitated by the Cancer Immunotherapy Consortium of the Cancer Research Institute and partner organizations systematically evaluated an immunotherapy-focused clinical development paradigm and created the principles for redefining trial endpoints. On this basis, a body of clinical and laboratory data was generated that supports three novel endpoint recommendations. First, cellular immune response assays generate highly variable results. Assay harmonization in multicenter trials may minimize variability and help to establish cellular immune response as a reproducible biomarker, thus allowing investigation of its relationship with clinical outcomes. Second, immunotherapy may induce novel patterns of antitumor response not captured by Response Evaluation Criteria in Solid Tumors or World Health Organization criteria. New immune-related response criteria were defined to more comprehensively capture all response patterns. Third, delayed separation of Kaplan-Meier curves in randomized immunotherapy trials can affect results. Altered statistical models describing hazard ratios as a function of time and recognizing differences before and after separation of curves may allow improved planning of phase III trials. These recommendations may improve our tools for cancer immunotherapy trials and may offer a more realistic and useful model for clinical investigation.
Figures

Comment in
-
The hazards of endpoints.J Natl Cancer Inst. 2010 Sep 22;102(18):1376-7. doi: 10.1093/jnci/djq334. Epub 2010 Sep 8. J Natl Cancer Inst. 2010. PMID: 20826734 No abstract available.
References
-
- Finke LH, Wentworth K, Blumenstein B, et al. Lessons from randomized phase III studies with active cancer immunotherapies—outcomes from the 2006 meeting of the Cancer Vaccine Consortium (CVC) Vaccine. 2007;25(suppl 2):B97–B109. - PubMed
-
- Copier J, Dalgleish AG, Britten CM, et al. Improving the efficacy of cancer immunotherapy. Eur J Cancer. 2009;45(8):1424–1431. - PubMed
-
- Finn OJ. Cancer immunology. N Engl J Med. 2008;358(25):2704–2715. - PubMed
-
- Melero I, Hervas-Stubbs S, Glennie M, et al. Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer. 2007;7(2):95–106. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

